A gateway-compatible yeast one-hybrid system
- PMID: 15489331
- PMCID: PMC528925
- DOI: 10.1101/gr.2445504
A gateway-compatible yeast one-hybrid system
Abstract
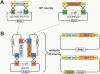
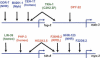
Since the advent of microarrays, vast amounts of gene expression data have been generated. However, these microarray data fail to reveal the transcription regulatory mechanisms that underlie differential gene expression, because the identity of the responsible transcription factors (TFs) often cannot be directly inferred from such data sets. Regulatory TFs activate or repress transcription of their _target genes by binding to cis-regulatory elements that are frequently located in a gene's promoter. To understand the mechanisms underlying differential gene expression, it is necessary to identify physical interactions between regulatory TFs and their _target genes. We developed a Gateway-compatible yeast one-hybrid (Y1H) system that enables the rapid, large-scale identification of protein-DNA interactions using both small (i.e., DNA elements of interest) and large (i.e., gene promoters) DNA fragments. We used four well-characterized Caenorhabditis elegans promoters as DNA baits to test the functionality of this Y1H system. We could detect approximately 40% of previously reported TF-promoter interactions. By performing screens using two complementary libraries, we found novel potentially interacting TFs for each promoter. We recapitulated several of the Y1H-based protein-DNA interactions using luciferase reporter assays in mammalian cells. Taken together, the Gateway-compatible Y1H system will allow the high-throughput identification of protein-DNA interactions and may be a valuable tool to decipher transcription regulatory networks.
Figures




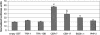
Similar articles
-
A gene-centered C. elegans protein-DNA interaction network.Cell. 2006 Jun 16;125(6):1193-205. doi: 10.1016/j.cell.2006.04.038. Cell. 2006. PMID: 16777607
-
Gateway-compatible yeast one-hybrid screens.CSH Protoc. 2006 Oct 1;2006(5):pdb.prot4590. doi: 10.1101/pdb.prot4590. CSH Protoc. 2006. PMID: 22485967
-
A high-throughput screening system for Arabidopsis transcription factors and its application to Med25-dependent transcriptional regulation.Mol Plant. 2011 May;4(3):546-55. doi: 10.1093/mp/ssr002. Epub 2011 Feb 22. Mol Plant. 2011. PMID: 21343311
-
Unraveling transcription regulatory networks by protein-DNA and protein-protein interaction mapping.Genome Res. 2006 Dec;16(12):1445-54. doi: 10.1101/gr.5321506. Epub 2006 Oct 19. Genome Res. 2006. PMID: 17053092 Review.
-
Gene-centered regulatory network mapping.Methods Cell Biol. 2011;106:271-88. doi: 10.1016/B978-0-12-544172-8.00010-4. Methods Cell Biol. 2011. PMID: 22118281 Free PMC article. Review.
Cited by
-
MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability.Nat Cell Biol. 2013 Jun;15(6):668-76. doi: 10.1038/ncb2741. Epub 2013 Apr 21. Nat Cell Biol. 2013. PMID: 23604316 Free PMC article.
-
Gene-centered regulatory networks.Brief Funct Genomics. 2010 Jan;9(1):4-12. doi: 10.1093/bfgp/elp049. Epub 2009 Dec 13. Brief Funct Genomics. 2010. PMID: 20008400 Free PMC article. Review.
-
Gateway compatible vectors for analysis of gene function in the zebrafish.Dev Dyn. 2007 Nov;236(11):3077-87. doi: 10.1002/dvdy.21354. Dev Dyn. 2007. PMID: 17948311 Free PMC article.
-
Insight into transcription factor gene duplication from Caenorhabditis elegans Promoterome-driven expression patterns.BMC Genomics. 2007 Jan 23;8:27. doi: 10.1186/1471-2164-8-27. BMC Genomics. 2007. PMID: 17244357 Free PMC article.
-
Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells.Proc Natl Acad Sci U S A. 2008 Jan 29;105(4):1380-5. doi: 10.1073/pnas.0711203105. Epub 2008 Jan 23. Proc Natl Acad Sci U S A. 2008. PMID: 18216250 Free PMC article.
References
-
- Adams, M.D., Celniker, S.E., Holt, R.A., Evans, C.A., Gocayne, J.D., Amanatides, P.G., Scherer, S.E., Li, P.W., Hoskins, R.A., Galle, R.F., et al. 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185-2195. - PubMed
-
- Boulton, S.J., Gartner, A., Reboul, J., Vaglio, P., Dyson, N., Hill, D.E., and Vidal, M. 2002. Combined functional genomic maps of the C. elegans DNA damage response. Science 295: 127-131. - PubMed
-
- The C. elegans Sequencing Consortium. 1998. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 282: 2012-2018. - PubMed
-
- Chen, P. and Ellis, R.E. 2000. TRA-1A regulates transcription of fog-3, which controls germ cell fate in C. elegans. Development 127: 3119-3129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
