Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model
- PMID: 15505083
- PMCID: PMC1851913
- DOI: 10.1161/01.CIR.0000147231.69595.D3
Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model
Abstract
Background: Computational biology is a powerful tool for elucidating arrhythmogenic mechanisms at the cellular level, where complex interactions between ionic processes determine behavior. A novel theoretical model of the canine ventricular epicardial action potential and calcium cycling was developed and used to investigate ionic mechanisms underlying Ca2+ transient (CaT) and action potential duration (APD) rate dependence.
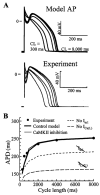
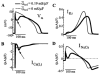
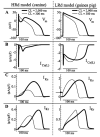
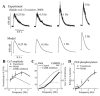
Methods and results: The Ca2+/calmodulin-dependent protein kinase (CaMKII) regulatory pathway was integrated into the model, which included a novel Ca2+-release formulation, Ca2+ subspace, dynamic chloride handling, and formulations for major ion currents based on canine ventricular data. Decreasing pacing cycle length from 8000 to 300 ms shortened APD primarily because of I(Ca(L)) reduction, with additional contributions from I(to1), I(NaK), and late I(Na). CaT amplitude increased as cycle length decreased from 8000 to 500 ms. This positive rate-dependent property depended on CaMKII activity.
Conclusions: CaMKII is an important determinant of the rate dependence of CaT but not of APD, which depends on ion-channel kinetics. The model of CaMKII regulation may serve as a paradigm for modeling effects of other regulatory pathways on cell function.
Figures

Similar articles
-
Regulation of Ca2+ and electrical alternans in cardiac myocytes: role of CAMKII and repolarizing currents.Am J Physiol Heart Circ Physiol. 2007 Jun;292(6):H2854-66. doi: 10.1152/ajpheart.01347.2006. Epub 2007 Feb 2. Am J Physiol Heart Circ Physiol. 2007. PMID: 17277017 Free PMC article.
-
Properties and ionic mechanisms of action potential adaptation, restitution, and accommodation in canine epicardium.Am J Physiol Heart Circ Physiol. 2009 Apr;296(4):H1017-26. doi: 10.1152/ajpheart.01216.2008. Epub 2009 Jan 23. Am J Physiol Heart Circ Physiol. 2009. PMID: 19168720 Free PMC article.
-
Molecular correlates of repolarization alternans in cardiac myocytes.J Mol Cell Cardiol. 2005 Sep;39(3):419-28. doi: 10.1016/j.yjmcc.2005.06.004. J Mol Cell Cardiol. 2005. PMID: 16026799
-
Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart.Cardiovasc Res. 2007 Mar 1;73(4):631-40. doi: 10.1016/j.cardiores.2006.11.005. Epub 2006 Nov 10. Cardiovasc Res. 2007. PMID: 17157285 Review.
-
Computational biology in the study of cardiac ion channels and cell electrophysiology.Q Rev Biophys. 2006 Feb;39(1):57-116. doi: 10.1017/S0033583506004227. Epub 2006 Jul 19. Q Rev Biophys. 2006. PMID: 16848931 Free PMC article. Review.
Cited by
-
Quantitative comparison of cardiac ventricular myocyte electrophysiology and response to drugs in human and nonhuman species.Am J Physiol Heart Circ Physiol. 2012 Mar 1;302(5):H1023-30. doi: 10.1152/ajpheart.00785.2011. Epub 2011 Dec 9. Am J Physiol Heart Circ Physiol. 2012. PMID: 22159993 Free PMC article.
-
Nonlinear dynamics in cardiology.Annu Rev Biomed Eng. 2012;14:179-203. doi: 10.1146/annurev-bioeng-071811-150106. Epub 2012 Apr 18. Annu Rev Biomed Eng. 2012. PMID: 22524390 Free PMC article. Review.
-
Modeling Calcium Cycling in the Heart: Progress, Pitfalls, and Challenges.Biomolecules. 2022 Nov 14;12(11):1686. doi: 10.3390/biom12111686. Biomolecules. 2022. PMID: 36421700 Free PMC article. Review.
-
Regulation of Ca2+ and electrical alternans in cardiac myocytes: role of CAMKII and repolarizing currents.Am J Physiol Heart Circ Physiol. 2007 Jun;292(6):H2854-66. doi: 10.1152/ajpheart.01347.2006. Epub 2007 Feb 2. Am J Physiol Heart Circ Physiol. 2007. PMID: 17277017 Free PMC article.
-
Mechanical regulation of gene expression in cardiac myocytes and fibroblasts.Nat Rev Cardiol. 2019 Jun;16(6):361-378. doi: 10.1038/s41569-019-0155-8. Nat Rev Cardiol. 2019. PMID: 30683889 Free PMC article. Review.
References
-
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. - PubMed
-
- Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. J Mol Cell Cardiol. 2002;34:919–939. - PubMed
-
- Hanson PI, Meyer T, Stryer L, Schulman H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–956. - PubMed
-
- Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol Heart Circ Physiol. 1994;267:H982–H993. - PubMed
-
- Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

