Novel roles for the AIDA adhesin from diarrheagenic Escherichia coli: cell aggregation and biofilm formation
- PMID: 15547278
- PMCID: PMC529095
- DOI: 10.1128/JB.186.23.8058-8065.2004
Novel roles for the AIDA adhesin from diarrheagenic Escherichia coli: cell aggregation and biofilm formation
Abstract
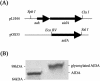
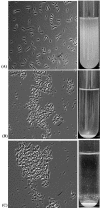
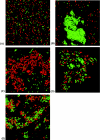
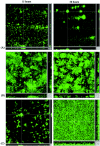
Diarrhea-causing Escherichia coli strains are responsible for numerous cases of gastrointestinal disease and constitute a serious health problem throughout the world. The ability to recognize and attach to host intestinal surfaces is an essential step in the pathogenesis of such strains. AIDA is a potent bacterial adhesin associated with some diarrheagenic E. coli strains. AIDA mediates bacterial attachment to a broad variety of human and other mammalian cells. It is a surface-displayed autotransporter protein and belongs to the selected group of bacterial glycoproteins; only the glycosylated form binds to mammalian cells. Here, we show that AIDA possesses self-association characteristics and can mediate autoaggregation of E. coli cells. We demonstrate that intercellular AIDA-AIDA interaction is responsible for bacterial autoaggregation. Interestingly, AIDA-expressing cells can interact with antigen 43 (Ag43)-expressing cells, which is indicative of an intercellular AIDA-Ag43 interaction. Additionally, AIDA expression dramatically enhances biofilm formation by E. coli on abiotic surfaces in flow chambers.
Figures


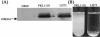
Similar articles
-
The TibA adhesin/invasin from enterotoxigenic Escherichia coli is self recognizing and induces bacterial aggregation and biofilm formation.Infect Immun. 2005 Apr;73(4):1954-63. doi: 10.1128/IAI.73.4.1954-1963.2005. Infect Immun. 2005. PMID: 15784535 Free PMC article.
-
Contribution of AIDA-I to the pathogenicity of a porcine diarrheagenic Escherichia coli and to intestinal colonization through biofilm formation in pigs.Vet Microbiol. 2007 Mar 10;120(3-4):308-19. doi: 10.1016/j.vetmic.2006.10.035. Epub 2006 Nov 30. Vet Microbiol. 2007. PMID: 17140750
-
Isolation and identification of AIDA-I receptors in porcine intestinal mucus.Vet Microbiol. 2008 Jan 25;126(4):345-55. doi: 10.1016/j.vetmic.2007.07.017. Epub 2007 Jul 25. Vet Microbiol. 2008. PMID: 17764859
-
Self-associating autotransporters, SAATs: functional and structural similarities.Int J Med Microbiol. 2006 Aug;296(4-5):187-95. doi: 10.1016/j.ijmm.2005.10.002. Int J Med Microbiol. 2006. PMID: 16600681 Review.
-
Adherence of diarrheagenic Escherichia coli strains to epithelial cells.Infect Immun. 2005 Jan;73(1):18-29. doi: 10.1128/IAI.73.1.18-29.2005. Infect Immun. 2005. PMID: 15618137 Free PMC article. Review. No abstract available.
Cited by
-
Molecular characterization of the EhaG and UpaG trimeric autotransporter proteins from pathogenic Escherichia coli.Appl Environ Microbiol. 2012 Apr;78(7):2179-89. doi: 10.1128/AEM.06680-11. Epub 2012 Jan 27. Appl Environ Microbiol. 2012. PMID: 22286983 Free PMC article.
-
Chlamydia trachomatis Polymorphic Membrane Proteins (Pmps) Form Functional Homomeric and Heteromeric Oligomers.Front Microbiol. 2021 Jul 19;12:709724. doi: 10.3389/fmicb.2021.709724. eCollection 2021. Front Microbiol. 2021. PMID: 34349750 Free PMC article.
-
In situ monitoring of IncF plasmid transfer on semi-solid agar surfaces reveals a limited invasion of plasmids in recipient colonies.Plasmid. 2012 Mar;67(2):155-61. doi: 10.1016/j.plasmid.2012.01.001. Epub 2012 Jan 10. Plasmid. 2012. PMID: 22248925 Free PMC article.
-
The Autotransporter IcsA Promotes Shigella flexneri Biofilm Formation in the Presence of Bile Salts.Infect Immun. 2019 Jun 20;87(7):e00861-18. doi: 10.1128/IAI.00861-18. Print 2019 Jul. Infect Immun. 2019. PMID: 30988059 Free PMC article.
-
Microaggregate-associated protein involved in invasion of epithelial cells by Mycobacterium avium subsp. hominissuis.Virulence. 2015;6(7):694-703. doi: 10.1080/21505594.2015.1072676. Virulence. 2015. PMID: 26252358 Free PMC article.
References
-
- Bachmann, B. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
-
- Benz, I., and M. A. Schmidt. 1992. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787(O126:H27), is synthesized via a precursor molecule. Mol. Microbiol. 6:1539-1546. - PubMed
-
- Benz, I., and M. A. Schmidt. 2001. Glycosylation with heptose residues mediated by the aah gene product is essential for adherence of the AIDA adhesin. Mol. Microbiol. 40:1403-1413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

