Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha
- PMID: 15569929
- PMCID: PMC534607
- DOI: 10.1073/pnas.0407492101
Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha
Abstract
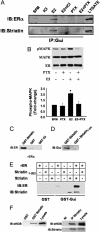
Steroid hormone receptors (SHRs) are ligand-activated transcription factors that regulate gene expression. SHRs also mediate rapid, nongenomic cellular activation by steroids. In vascular endothelial cells, the SHR for estrogen, estrogen receptor (ER) alpha, is _targeted by unknown mechanisms to a functional signaling module in membrane caveolae that enables estrogen to rapidly activate the mitogen-activated protein kinase and phosphatidylinositol 3-Akt kinase pathways, and endothelial NO synthase (eNOS). Here we identify the 110-kDa caveolin-binding protein striatin as the molecular anchor that localizes ERalpha to the membrane and organizes the ERalpha-eNOS membrane signaling complex. Striatin directly binds to amino acids 183-253 of ERalpha, _targets ERalpha to the cell membrane, and serves as a scaffold for the formation of an ERalpha-Galphai complex. Disruption of complex formation between ERalpha and striatin blocks estrogen-induced rapid activation mitogen-activated protein kinase, Akt kinase, and eNOS, but has no effect on ER-dependent regulation of an estrogen response element-driven reporter plasmid. These findings identify striatin as a molecular scaffold required for rapid, nongenomic estrogen-mediated activation of downstream signaling pathways. Furthermore, by demonstrating independent regulation of nongenomic vs. genomic ER-dependent signaling, these findings provide conceptual support for the potential development of "pathway-specific" selective ER modulators.
Figures
Similar articles
-
Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae.Circ Res. 2000 Nov 24;87(11):E44-52. doi: 10.1161/01.res.87.11.e44. Circ Res. 2000. PMID: 11090554
-
Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen.J Clin Invest. 1999 Feb;103(3):401-6. doi: 10.1172/JCI5347. J Clin Invest. 1999. PMID: 9927501 Free PMC article.
-
Aldosterone's rapid, nongenomic effects are mediated by striatin: a modulator of aldosterone's effect on estrogen action.Endocrinology. 2014 Jun;155(6):2233-43. doi: 10.1210/en.2013-1834. Epub 2014 Mar 21. Endocrinology. 2014. PMID: 24654783 Free PMC article.
-
Estrogen modulation of endothelial nitric oxide synthase.Endocr Rev. 2002 Oct;23(5):665-86. doi: 10.1210/er.2001-0045. Endocr Rev. 2002. PMID: 12372846 Review.
-
Rapid activation of endothelial NO synthase by estrogen: evidence for a steroid receptor fast-action complex (SRFC) in caveolae.Steroids. 2002 May;67(6):413-9. doi: 10.1016/s0039-128x(01)00177-5. Steroids. 2002. PMID: 11960616 Review.
Cited by
-
Rapid estrogen receptor signaling mediates estrogen-induced inhibition of vascular smooth muscle cell proliferation.Arterioscler Thromb Vasc Biol. 2013 Aug;33(8):1837-43. doi: 10.1161/ATVBAHA.112.300752. Epub 2013 Jun 6. Arterioscler Thromb Vasc Biol. 2013. PMID: 23744991 Free PMC article.
-
Point mutations in the ERα Gαi binding domain segregate nonnuclear from nuclear receptor function.Mol Endocrinol. 2013 Jan;27(1):2-11. doi: 10.1210/me.2011-1378. Epub 2012 Dec 14. Mol Endocrinol. 2013. PMID: 23242705 Free PMC article.
-
Progesterone-estrogen interactions in synaptic plasticity and neuroprotection.Neuroscience. 2013 Jun 3;239:280-94. doi: 10.1016/j.neuroscience.2012.10.051. Epub 2012 Nov 7. Neuroscience. 2013. PMID: 23142339 Free PMC article. Review.
-
Precise and systematic survey of the efficacy of multicomponent drugs against functional dyspepsia.Sci Rep. 2019 Jul 24;9(1):10713. doi: 10.1038/s41598-019-47300-7. Sci Rep. 2019. PMID: 31341240 Free PMC article.
-
Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications.Steroids. 2007 May;72(5):381-405. doi: 10.1016/j.steroids.2007.02.003. Epub 2007 Feb 21. Steroids. 2007. PMID: 17379265 Free PMC article. Review.
References
-
- Mendelsohn, M. E. & Karas, R. H. (1999) N. Engl. J. Med. 340, 1801–1811. - PubMed
-
- Chambliss, K. L., Yuhanna, I. S., Anderson, R. G., Mendelsohn, M. E. & Shaul, P. W. (2002) Mol. Endocrinol. 16, 938–946. - PubMed
-
- Chambliss, K. L., Yuhannna, I. S., Mineo, C., Liu, C., German, Z., Sherman, T. S., Mendelsohn, M. E., Anderson, R. G. W. & Shaul, P. W. (2000) Circ. Res. E44–E52. - PubMed
-
- Haynes, M. P., Sinha, D., Strong Russell, K., Collinge, M., Fulton D, MoralesRuiz, M. & Bender, J. R. (2000) Circ. Res. 87, 677–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

