CaV2.3 channel and PKClambda: new players in insulin secretion
- PMID: 15630435
- PMCID: PMC539207
- DOI: 10.1172/JCI23970
CaV2.3 channel and PKClambda: new players in insulin secretion
Abstract
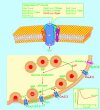
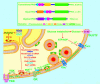
Insulin secretion is critically dependent on the proper function of a complex molecular network. Ca(V)2.3-knockout (Ca(V)2.3(-/-)) and PKClambda-knockout (PKClambda(-/-)) mouse models now suggest that these 2 players, the Ca(v)2.3 channel and PKClambda, are important constituents of this molecular network. Subsequent to glucose stimulation, insulin is released from the pancreatic beta cell in a biphasic pattern, i.e., a rapid initial phase followed by a slower, more sustained phase. Interestingly, Ca(2+) influx through the Ca(V)2.3 channel regulates only the second phase of insulin secretion. PKClambda seems to enter the beta cell nucleus and in turn modulates the expression of several genes critical for beta cell secretory function. Studies by Hashimoto et al. and Jing et al. in this issue of the JCI set out to answer the question of why numerous isoforms of proteins with similar functions are present in the beta cell. This is important, since it has been difficult to understand the modulatory and/or regulatory roles of different isoforms of proteins in defined subcellular compartments and at various times during the secretory process in both beta cell physiology and pathophysiology.
Figures
Comment on
-
PKClambda regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic beta cells.J Clin Invest. 2005 Jan;115(1):138-45. doi: 10.1172/JCI22232. J Clin Invest. 2005. PMID: 15630453 Free PMC article.
-
CaV2.3 calcium channels control second-phase insulin release.J Clin Invest. 2005 Jan;115(1):146-54. doi: 10.1172/JCI22518. J Clin Invest. 2005. PMID: 15630454 Free PMC article.
Similar articles
-
CaV2.3 calcium channels control second-phase insulin release.J Clin Invest. 2005 Jan;115(1):146-54. doi: 10.1172/JCI22518. J Clin Invest. 2005. PMID: 15630454 Free PMC article.
-
PKClambda regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic beta cells.J Clin Invest. 2005 Jan;115(1):138-45. doi: 10.1172/JCI22232. J Clin Invest. 2005. PMID: 15630453 Free PMC article.
-
Diethyldithiocarbamate-mediated zinc ion chelation reveals role of Cav2.3 channels in glucagon secretion.Biochim Biophys Acta. 2015 May;1853(5):953-64. doi: 10.1016/j.bbamcr.2015.01.001. Epub 2015 Jan 17. Biochim Biophys Acta. 2015. PMID: 25603538
-
Regulation of insulin secretion in islets of Langerhans by Ca(2+)channels.J Membr Biol. 2004 Jul 15;200(2):57-66. doi: 10.1007/s00232-004-0692-9. J Membr Biol. 2004. PMID: 15520904 Review.
-
[The alpha 1 subunit of voltage-dependent calcium channel expressed in pancreatic beta cells].Nihon Rinsho. 1994 Oct;52(10):2593-8. Nihon Rinsho. 1994. PMID: 7983784 Review. Japanese.
Cited by
-
The anterior chamber of the eye technology and its anatomical, optical, and immunological bases.Physiol Rev. 2024 Jul 1;104(3):881-929. doi: 10.1152/physrev.00024.2023. Epub 2024 Jan 11. Physiol Rev. 2024. PMID: 38206586 Free PMC article. Review.
-
Association Study of CACNA1D, KCNJ11, KCNQ1, and CACNA1E Single-Nucleotide Polymorphisms with Type 2 Diabetes Mellitus.Int J Mol Sci. 2024 Aug 24;25(17):9196. doi: 10.3390/ijms25179196. Int J Mol Sci. 2024. PMID: 39273144 Free PMC article.
-
CACNA1E variants affect beta cell function in patients with newly diagnosed type 2 diabetes. the Verona newly diagnosed type 2 diabetes study (VNDS) 3.PLoS One. 2012;7(3):e32755. doi: 10.1371/journal.pone.0032755. Epub 2012 Mar 9. PLoS One. 2012. PMID: 22427875 Free PMC article.
-
Pancreatic Crosstalk in the Disease Setting: Understanding the Impact of Exocrine Disease on Endocrine Function.Compr Physiol. 2024 Mar 29;14(2):5371-5387. doi: 10.1002/cphy.c230008. Compr Physiol. 2024. PMID: 39109973 Free PMC article. Review.
-
Metabolic syndrome induces changes in KATP-channels and calcium currents in pancreatic β-cells.Islets. 2012 Jul-Aug;4(4):302-11. doi: 10.4161/isl.21374. Epub 2012 Jul 1. Islets. 2012. PMID: 22885660 Free PMC article.
References
-
- Yang S-N, Berggren P-O. β-Cell CaV channel regulation in physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2005;288:E16–E28. - PubMed
-
- Aizawa T, Komatsu M, Asanuma N, Sato Y, Sharp GW. Glucose action ‘beyond ionic events’ in the pancreatic β cell. Trends Pharmacol. Sci. 1998;19:496–499. - PubMed
-
- Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab. Res. Rev. 2002;18:451–463. - PubMed
-
- Nesher R, Cerasi E. Biphasic insulin release as the expression of combined inhibitory and potentiating effects of glucose. Endocrinology. 1987;121:1017–1024. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

