Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes
- PMID: 15681609
- PMCID: PMC548946
- DOI: 10.1101/gad.1263305
Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes
Abstract
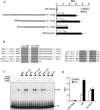
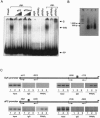
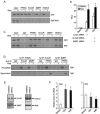
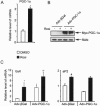
Peroxisome proliferator-activated receptor gamma (PPARgamma) is the master regulator of adipogenesis as well as the _target of thiazolidinedione (TZD) antidiabetic drugs. Many PPARgamma _target genes are induced during adipogenesis, but others, such as glycerol kinase (GyK), are expressed at low levels in adipocytes and dramatically up-regulated by TZDs. Here, we have explored the mechanism whereby an exogenous PPARgamma ligand is selectively required for adipocyte gene expression. The GyK gene contains a functional PPARgamma-response element to which endogenous PPARgamma is recruited in adipocytes. However, unlike the classic PPARgamma-_target gene aP2, which is constitutively associated with coactivators, the GyK gene is _targeted by nuclear receptor corepressors in adipocytes. TZDs trigger the dismissal of corepressor histone deacetylase (HDAC) complexes and the recruitment of coactivators to the GyK gene. TZDs also induce PPARgamma-Coactivator 1alpha (PGC-1alpha), whose recruitment to the GyK gene is sufficient to release the corepressors. Thus, selective modulation of adipocyte PPARgamma _target genes by TZDs involves the dissociation of corepressors by direct and indirect mechanisms.
Figures


Comment in
-
'No, really, how do they work?'.Genes Dev. 2005 Feb 15;19(4):413-4. doi: 10.1101/gad.1294105. Genes Dev. 2005. PMID: 15713837 No abstract available.
Similar articles
-
The transcriptional coactivator peroxisome proliferator activated receptor (PPAR)gamma coactivator-1 alpha and the nuclear receptor PPAR alpha control the expression of glycerol kinase and metabolism genes independently of PPAR gamma activation in human white adipocytes.Diabetes. 2007 Oct;56(10):2467-75. doi: 10.2337/db06-1465. Epub 2007 Jul 23. Diabetes. 2007. PMID: 17646210
-
Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1alpha gene transcription: an autoregulatory loop controls PGC-1alpha expression in adipocytes via peroxisome proliferator-activated receptor-gamma coactivation.Endocrinology. 2006 Jun;147(6):2829-38. doi: 10.1210/en.2006-0070. Epub 2006 Mar 2. Endocrinology. 2006. PMID: 16513826
-
A peroxisome proliferator-activated receptor gamma (PPARgamma)/PPARgamma coactivator 1beta autoregulatory loop in adipocyte mitochondrial function.J Biol Chem. 2011 Sep 2;286(35):30723-30731. doi: 10.1074/jbc.M111.251926. Epub 2011 Jun 30. J Biol Chem. 2011. PMID: 21719705 Free PMC article.
-
[PPARgamma _target genes and the molecular mechanism of transcriptional control by PPARgamma].Nihon Rinsho. 2010 Feb;68(2):189-93. Nihon Rinsho. 2010. PMID: 20158083 Review. Japanese.
-
Transcriptional Regulatory Circuits Controlling Brown Fat Development and Activation.Diabetes. 2015 Jul;64(7):2369-75. doi: 10.2337/db15-0203. Epub 2015 Jun 7. Diabetes. 2015. PMID: 26050669 Free PMC article. Review.
Cited by
-
Biochemical adaptations of mammalian hibernation: exploring squirrels as a perspective model for naturally induced reversible insulin resistance.Braz J Med Biol Res. 2013 Jan;46(1):1-13. doi: 10.1590/1414-431x20122388. Epub 2013 Jan 11. Braz J Med Biol Res. 2013. PMID: 23314346 Free PMC article. Review.
-
The silencing mediator of retinoid and thyroid hormone receptors (SMRT) regulates adipose tissue accumulation and adipocyte insulin sensitivity in vivo.J Biol Chem. 2010 Jun 11;285(24):18485-95. doi: 10.1074/jbc.M110.107680. Epub 2010 Apr 6. J Biol Chem. 2010. PMID: 20371609 Free PMC article.
-
Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes.Mol Cell Biol. 2008 Jan;28(1):188-200. doi: 10.1128/MCB.00992-07. Epub 2007 Oct 22. Mol Cell Biol. 2008. PMID: 17954559 Free PMC article.
-
Parvin-beta inhibits breast cancer tumorigenicity and promotes CDK9-mediated peroxisome proliferator-activated receptor gamma 1 phosphorylation.Mol Cell Biol. 2008 Jan;28(2):687-704. doi: 10.1128/MCB.01617-06. Epub 2007 Nov 12. Mol Cell Biol. 2008. PMID: 17998334 Free PMC article.
-
Further understanding of fat biology: lessons from a fat fly.Exp Mol Med. 2010 Jan 31;42(1):12-20. doi: 10.3858/emm.2010.42.1.007. Exp Mol Med. 2010. PMID: 19887892 Free PMC article. Review.
References
-
- Adams M., Reginato, M.J., Shao, D., Lazar, M.A., and Chatterjee, V.K. 1997. Transcriptional activation by PPARγ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J. Biol. Chem. 272: 5128–5132. - PubMed
-
- Armoni M., Kritz, N., Harel, C., Bar-Yoseph, F., Chen, H., Quon, M.J., and Karnieli, E. 2003. Peroxisome proliferator-activated receptor-γ represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J. Biol. Chem. 278: 30614–30623. - PubMed
-
- Camp H.S., Chaudhry, A., and Leff, T. 2001. A novel potent antagonist of peroxisome proliferator-activated receptor γ blocks adipocyte differentiation but does not revert the phenotype of terminally differentiated adipocytes. Endocrinology 142: 3207–3213. - PubMed
-
- Chawla A., Schwarz, E.J., Dimaculangan, D.D., and Lazar, M.A. 1994. Peroxisome proliferator-activated receptor γ (PPARγ): Adipose predominant expression and induction early in adipocyte differentiation. Endocrinology 135: 798–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
