A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers
- PMID: 15932946
- PMCID: PMC1157030
- DOI: 10.1073/pnas.0501112102
A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers
Abstract
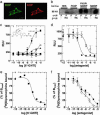
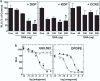
There has been much speculation regarding the functional relevance of G protein-coupled receptor heterodimers, primarily because demonstrating their existence in vivo has proven to be a considerable challenge. Here we show that the opioid agonist ligand 6'-guanidinonaltrindole (6'-GNTI) has the unique property of selectively activating only opioid receptor heterodimers but not homomers. Importantly, 6'-GNTI is an analgesic, thereby demonstrating that opioid receptor heterodimers are indeed functionally relevant in vivo. However, 6'-GNTI induces analgesia only when it is administered in the spinal cord but not in the brain, suggesting that the organization of heterodimers is tissue-specific. This study demonstrates a proof of concept for tissue-selective drug _targeting based on G protein-coupled receptor heterodimerization. Importantly, _targeting opioid heterodimers could provide an approach toward the design of analgesic drugs with reduced side effects.
Figures

Comment in
-
Diversifying the repertoire of G protein-coupled receptors through oligomerization.Proc Natl Acad Sci U S A. 2005 Jun 21;102(25):8793-4. doi: 10.1073/pnas.0504016102. Epub 2005 Jun 13. Proc Natl Acad Sci U S A. 2005. PMID: 15956197 Free PMC article. No abstract available.
Similar articles
-
5'-Guanidinonaltrindole, a highly selective and potent kappa-opioid receptor antagonist.Eur J Pharmacol. 2000 May 12;396(1):49-52. doi: 10.1016/s0014-2999(00)00208-9. Eur J Pharmacol. 2000. PMID: 10822054
-
Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system.J Pharmacol Exp Ther. 2002 Nov;303(2):549-56. doi: 10.1124/jpet.102.037861. J Pharmacol Exp Ther. 2002. PMID: 12388635
-
Interactions among mu- and delta-opioid receptors, especially putative delta1- and delta2-opioid receptors, promote dopamine release in the nucleus accumbens.Neuroscience. 2005;135(1):213-25. doi: 10.1016/j.neuroscience.2005.03.065. Neuroscience. 2005. PMID: 16111831
-
_targeting opioid receptor heterodimers: strategies for screening and drug development.AAPS J. 2006 Mar 10;8(1):E153-9. doi: 10.1208/aapsj080118. AAPS J. 2006. PMID: 16584123 Free PMC article. Review.
-
G protein-coupled receptor dimerization: function and ligand pharmacology.Mol Pharmacol. 2004 Jul;66(1):1-7. doi: 10.1124/mol.104.000497.. Mol Pharmacol. 2004. PMID: 15213289 Review.
Cited by
-
Dynamic models of G-protein coupled receptor dimers: indications of asymmetry in the rhodopsin dimer from molecular dynamics simulations in a POPC bilayer.J Comput Aided Mol Des. 2006 Jul-Aug;20(7-8):405-16. doi: 10.1007/s10822-006-9053-3. Epub 2006 Nov 7. J Comput Aided Mol Des. 2006. PMID: 17089205 Free PMC article.
-
Taking two to tango: a role for ghrelin receptor heterodimerization in stress and reward.Front Neurosci. 2013 Aug 30;7:148. doi: 10.3389/fnins.2013.00148. Front Neurosci. 2013. PMID: 24009547 Free PMC article. Review.
-
Biological redundancy of endogenous GPCR ligands in the gut and the potential for endogenous functional selectivity.Front Pharmacol. 2014 Nov 28;5:262. doi: 10.3389/fphar.2014.00262. eCollection 2014. Front Pharmacol. 2014. PMID: 25506328 Free PMC article. Review.
-
Antibodies to probe endogenous G protein-coupled receptor heteromer expression, regulation, and function.Front Pharmacol. 2014 Dec 3;5:268. doi: 10.3389/fphar.2014.00268. eCollection 2014. Front Pharmacol. 2014. PMID: 25520661 Free PMC article. Review.
-
Native serotonin 5-HT2C receptors are expressed as homodimers on the apical surface of choroid plexus epithelial cells.Mol Pharmacol. 2015 Apr;87(4):660-73. doi: 10.1124/mol.114.096636. Epub 2015 Jan 21. Mol Pharmacol. 2015. PMID: 25609374 Free PMC article.
References
-
- Pin, J. P., Kniazeff, J., Binet, V., Liu, J., Maurel, D., Galvez, T., Duthey, B., Havlickova, M., Blahos, J., Prezeau, L. & Rondard, P. (2004) Biochem. Pharmacol. 68, 1565–1572. - PubMed
-
- Kniazeff, J., Bessis, A. S., Maurel, D., Ansanay, H., Prezeau, L. & Pin, J. P. (2004) Nat. Struct. Mol. Biol. 11, 706–713. - PubMed
-
- Nelson, G., Chandrashekar, J., Hoon, M. A., Feng, L., Zhao, G., Ryba, N. J. & Zuker, C. S. (2002) Nature 416, 199–202. - PubMed
-
- Filipek, S., Krzysko, K. A., Fotiadis, D., Liang, Y., Saperstein, D. A., Engel, A. & Palczewski, K. (2004) Photochem. Photobiol. Sci. 3, 628–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

