Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis
- PMID: 16079851
- PMCID: PMC1939938
- DOI: 10.1038/nature03918
Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis
Abstract
Cellular senescence has been theorized to oppose neoplastic transformation triggered by activation of oncogenic pathways in vitro, but the relevance of senescence in vivo has not been established. The PTEN and p53 tumour suppressors are among the most commonly inactivated or mutated genes in human cancer including prostate cancer. Although they are functionally distinct, reciprocal cooperation has been proposed, as PTEN is thought to regulate p53 stability, and p53 to enhance PTEN transcription. Here we show that conditional inactivation of Trp53 in the mouse prostate fails to produce a tumour phenotype, whereas complete Pten inactivation in the prostate triggers non-lethal invasive prostate cancer after long latency. Strikingly, combined inactivation of Pten and Trp53 elicits invasive prostate cancer as early as 2 weeks after puberty and is invariably lethal by 7 months of age. Importantly, acute Pten inactivation induces growth arrest through the p53-dependent cellular senescence pathway both in vitro and in vivo, which can be fully rescued by combined loss of Trp53. Furthermore, we detected evidence of cellular senescence in specimens from early-stage human prostate cancer. Our results demonstrate the relevance of cellular senescence in restricting tumorigenesis in vivo and support a model for cooperative tumour suppression in which p53 is an essential failsafe protein of Pten-deficient tumours.
Figures
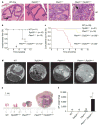
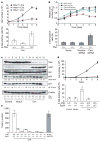
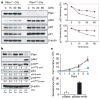
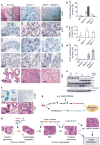
Comment in
-
Cancer: crime and punishment.Nature. 2005 Aug 4;436(7051):636-7. doi: 10.1038/436636a. Nature. 2005. PMID: 16079829 No abstract available.
Similar articles
-
Cancer: crime and punishment.Nature. 2005 Aug 4;436(7051):636-7. doi: 10.1038/436636a. Nature. 2005. PMID: 16079829 No abstract available.
-
Skp2 _targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence.Nature. 2010 Mar 18;464(7287):374-9. doi: 10.1038/nature08815. Nature. 2010. PMID: 20237562 Free PMC article.
-
Genetic analysis of Pten and Ink4a/Arf interactions in the suppression of tumorigenesis in mice.Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1455-60. doi: 10.1073/pnas.022632099. Epub 2002 Jan 29. Proc Natl Acad Sci U S A. 2002. PMID: 11818530 Free PMC article.
-
PTEN: a default gate-keeping tumor suppressor with a versatile tail.Cell Res. 2008 Aug;18(8):807-16. doi: 10.1038/cr.2008.83. Cell Res. 2008. PMID: 18626510 Review.
-
The role of PTEN in the progression and survival of prostate cancer.Minerva Endocrinol. 2003 Jun;28(2):145-53. Minerva Endocrinol. 2003. PMID: 12717346 Review.
Cited by
-
Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells.Cancer Res. 2012 Apr 1;72(7):1878-89. doi: 10.1158/0008-5472.CAN-11-3132. Epub 2012 Feb 20. Cancer Res. 2012. PMID: 22350410 Free PMC article.
-
Doxycycline regulated induction of AKT in murine prostate drives proliferation independently of p27 cyclin dependent kinase inhibitor downregulation.PLoS One. 2012;7(7):e41330. doi: 10.1371/journal.pone.0041330. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844460 Free PMC article.
-
Role of Ubiquitination in PTEN Cellular Homeostasis and Its Implications in GB Drug Resistance.Front Oncol. 2020 Sep 2;10:1569. doi: 10.3389/fonc.2020.01569. eCollection 2020. Front Oncol. 2020. PMID: 32984016 Free PMC article. Review.
-
Impact of PTEN abnormalities on outcome in pediatric patients with T-cell acute lymphoblastic leukemia treated on the MRC UKALL2003 trial.Leukemia. 2016 Jan;30(1):39-47. doi: 10.1038/leu.2015.206. Epub 2015 Jul 29. Leukemia. 2016. PMID: 26220040 Free PMC article.
-
RAS, cellular senescence and transformation: the BRCA1 DNA repair pathway at the crossroads.Small GTPases. 2012 Jul-Sep;3(3):163-7. doi: 10.4161/sgtp.19884. Epub 2012 Jul 1. Small GTPases. 2012. PMID: 22751483 Free PMC article.
References
-
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. - PubMed
-
- Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol. 2001;13:748–753. - PubMed
-
- Campisi J. Cellular senescence as a tumour-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. - PubMed
-
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. - PubMed
-
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumour suppression. Cell. 2000;100:387–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
