Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry
- PMID: 16081529
- PMCID: PMC1188015
- DOI: 10.1073/pnas.0505577102
Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry
Abstract
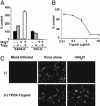
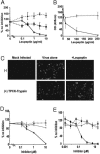
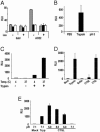
Severe acute respiratory syndrome (SARS) is caused by an emergent coronavirus (SARS-CoV), for which there is currently no effective treatment. SARS-CoV mediates receptor binding and entry by its spike (S) glycoprotein, and infection is sensitive to lysosomotropic agents that perturb endosomal pH. We demonstrate here that the lysosomotropic-agent-mediated block to SARS-CoV infection is overcome by protease treatment of _target-cell-associated virus. In addition, SARS-CoV infection was blocked by specific inhibitors of the pH-sensitive endosomal protease cathepsin L. A cell-free membrane-fusion system demonstrates that engagement of receptor followed by proteolysis is required for SARS-CoV membrane fusion and indicates that cathepsin L is sufficient to activate membrane fusion by SARS-CoV S. These results suggest that SARS-CoV infection results from a unique, three-step process: receptor binding and induced conformational changes in S glycoprotein followed by cathepsin L proteolysis within endosomes. The requirement for cathepsin L proteolysis identifies a previously uncharacterized class of inhibitor for SARS-CoV infection.
Figures
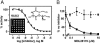
Similar articles
-
Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level.Am J Respir Cell Mol Biol. 2008 Aug;39(2):142-9. doi: 10.1165/rcmb.2007-0217OC. Epub 2008 Feb 28. Am J Respir Cell Mol Biol. 2008. PMID: 18314540
-
SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells.J Biol Chem. 2006 Feb 10;281(6):3198-203. doi: 10.1074/jbc.M508381200. Epub 2005 Dec 8. J Biol Chem. 2006. PMID: 16339146 Free PMC article.
-
Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry.J Virol. 2006 Jun;80(12):5768-76. doi: 10.1128/JVI.00442-06. J Virol. 2006. PMID: 16731916 Free PMC article.
-
Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research.Antiviral Res. 2013 Dec;100(3):605-14. doi: 10.1016/j.antiviral.2013.09.028. Epub 2013 Oct 8. Antiviral Res. 2013. PMID: 24121034 Free PMC article. Review.
-
SARS coronavirus anti-infectives.Recent Pat Antiinfect Drug Discov. 2006 Nov;1(3):297-308. doi: 10.2174/157489106778777637. Recent Pat Antiinfect Drug Discov. 2006. PMID: 18221155 Review.
Cited by
-
Cell Entry of Porcine Epidemic Diarrhea Coronavirus Is Activated by Lysosomal Proteases.J Biol Chem. 2016 Nov 18;291(47):24779-24786. doi: 10.1074/jbc.M116.740746. Epub 2016 Oct 11. J Biol Chem. 2016. PMID: 27729455 Free PMC article.
-
Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2.Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20803-20813. doi: 10.1073/pnas.2007837117. Epub 2020 Aug 6. Proc Natl Acad Sci U S A. 2020. PMID: 32764148 Free PMC article.
-
Melatonin potentials against viral infections including COVID-19: Current evidence and new findings.Virus Res. 2020 Oct 2;287:198108. doi: 10.1016/j.virusres.2020.198108. Epub 2020 Aug 5. Virus Res. 2020. PMID: 32768490 Free PMC article. Review.
-
Peptides: Prospects for Use in the Treatment of COVID-19.Molecules. 2020 Sep 24;25(19):4389. doi: 10.3390/molecules25194389. Molecules. 2020. PMID: 32987757 Free PMC article. Review.
-
Broad-spectrum antiviral agents.Front Microbiol. 2015 May 22;6:517. doi: 10.3389/fmicb.2015.00517. eCollection 2015. Front Microbiol. 2015. PMID: 26052325 Free PMC article. Review.
References
-
- Rota, P. A., Oberste, M. S., Monroe, S. S., Nix, W. A., Campagnoli, R., Icenogle, J. P., Penaranda, S., Bankamp, B., Maher, K., Chen, M. H., et al. (2003) Science 300, 1394-1399. - PubMed
-
- Guan, Y., Zheng, B. J., He, Y. Q., Liu, X. L., Zhuang, Z. X., Cheung, C. L., Luo, S. W., Li, P. H., Zhang, L. J., Guan, Y. J., et al. (2003) Science 302, 276-278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

