Elevated expression of MITF counteracts B-RAF-stimulated melanocyte and melanoma cell proliferation
- PMID: 16129781
- PMCID: PMC2171350
- DOI: 10.1083/jcb.200505059
Elevated expression of MITF counteracts B-RAF-stimulated melanocyte and melanoma cell proliferation
Abstract
The protein kinase B-RAF is a human oncogene that is mutated in approximately 70% of human melanomas and transforms mouse melanocytes. Microphthalmia-associated transcription factor (MITF) is an important melanocyte differentiation and survival factor, but its role in melanoma is unclear. In this study, we show that MITF expression is suppressed by oncogenic B-RAF in immortalized mouse and primary human melanocytes. However, low levels of MITF persist in human melanoma cells harboring oncogenic B-RAF, suggesting that additional mechanisms regulate its expression. MITF reexpression in B-RAF-transformed melanocytes inhibits their proliferation. Furthermore, differentiation-inducing factors that elevate MITF expression in melanoma cells inhibit their proliferation, but when MITF up-regulation is prevented by RNA interference, proliferation is not inhibited. These data suggest that MITF is an anti-proliferation factor that is down-regulated by B-RAF signaling and that this is a crucial event for the progression of melanomas that harbor oncogenic B-RAF.
Figures
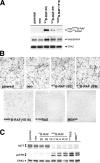
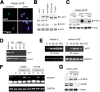
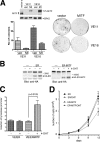
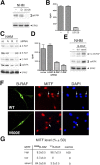
Similar articles
-
Hypoxia-inducible factor 1{alpha} is a new _target of microphthalmia-associated transcription factor (MITF) in melanoma cells.J Cell Biol. 2005 Jul 4;170(1):49-59. doi: 10.1083/jcb.200501067. Epub 2005 Jun 27. J Cell Biol. 2005. PMID: 15983061 Free PMC article.
-
ERK-regulated differential expression of the Mitf 6a/b splicing isoforms in melanoma.Pigment Cell Melanoma Res. 2010 Feb;23(1):93-102. doi: 10.1111/j.1755-148X.2009.00652.x. Epub 2009 Nov 6. Pigment Cell Melanoma Res. 2010. PMID: 19895547
-
The melanocyte-specific isoform of the microphthalmia transcription factor affects the phenotype of human melanoma.Cancer Res. 2002 Apr 1;62(7):2098-103. Cancer Res. 2002. PMID: 11929831
-
[Malignant melanoma and the role of the paradoxal protein Microphthalmia transcription factor].Bull Cancer. 2007 Jan;94(1):81-92. Bull Cancer. 2007. PMID: 17237008 Review. French.
-
Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival.Oncogene. 2003 May 19;22(20):3035-41. doi: 10.1038/sj.onc.1206443. Oncogene. 2003. PMID: 12789278 Review.
Cited by
-
Effect of SMURF2 _targeting on susceptibility to MEK inhibitors in melanoma.J Natl Cancer Inst. 2013 Jan 2;105(1):33-46. doi: 10.1093/jnci/djs471. Epub 2012 Dec 17. J Natl Cancer Inst. 2013. PMID: 23250956 Free PMC article.
-
Main roads to melanoma.J Transl Med. 2009 Oct 14;7:86. doi: 10.1186/1479-5876-7-86. J Transl Med. 2009. PMID: 19828018 Free PMC article. Review.
-
A PAX3/BRN2 rheostat controls the dynamics of BRAF mediated MITF regulation in MITFhigh /AXLlow melanoma.Pigment Cell Melanoma Res. 2019 Mar;32(2):280-291. doi: 10.1111/pcmr.12741. Epub 2018 Oct 19. Pigment Cell Melanoma Res. 2019. PMID: 30277012 Free PMC article.
-
Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway _targeted therapy.Pigment Cell Melanoma Res. 2015 Jul;28(4):390-406. doi: 10.1111/pcmr.12370. Epub 2015 Apr 17. Pigment Cell Melanoma Res. 2015. PMID: 25818589 Free PMC article. Review.
-
Somatic mutations in solid tumors: a spectrum at the service of diagnostic armamentarium or an indecipherable puzzle? The morphological eyes looking for BRAF and somatic molecular detections on cyto-histological samples.Onco_target. 2017 Jan 10;8(2):3746-3760. doi: 10.18632/onco_target.12564. Onco_target. 2017. PMID: 27738305 Free PMC article. Review.
References
-
- Carreira, S., J. Goodall, I. Aksan, S.A. La Rocca, M.D. Galibert, L. Denat, L. Larue, and C.R. Goding. 2005. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature. 433:764–769. - PubMed
-
- Davies, H., G.R. Bignell, C. Cox, P. Stephens, S. Edkins, S. Clegg, J. Teague, H. Woffendin, M.J. Garnett, W. Bottomley, et al. 2002. Mutations of the BRAF gene in human cancer. Nature. 417:949–954. - PubMed
-
- Du, J., H.R. Widlund, M.A. Horstmann, S. Ramaswamy, K. Ross, W.E. Huber, E.K. Nishimura, T.R. Golub, and D.E. Fisher. 2004. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 6:565–576. - PubMed
-
- Duncan, L.M., J. Deeds, J. Hunter, J. Shao, L.M. Holmgren, E.A. Woolf, R.I. Tepper, and A.W. Shyjan. 1998. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 58:1515–1520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

