Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction
- PMID: 16260617
- PMCID: PMC1280268
- DOI: 10.1128/MCB.25.22.10040-10051.2005
Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction
Abstract
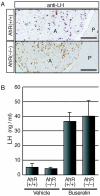
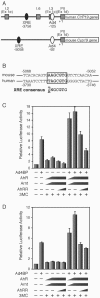
Dioxins exert a variety of adverse effects on organisms, including teratogenesis, immunosuppression, tumor promotion, and estrogenic action. Studies using aryl hydrocarbon receptor (AhR)-deficient mice suggest that the majority of these toxic effects are mediated by the AhR. In spite of the adverse effects mediated by this receptor, the AhR gene is conserved among a number of animal species, ranging from invertebrates to vertebrates. This high degree of conservation strongly suggests that AhR possesses an important physiologic function, and a critical function is also supported by the reduced fertility observed with AhR-null female mice. We demonstrate that AhR plays a crucial role in female reproduction by regulating the expression of ovarian P450 aromatase (Cyp19), a key enzyme in estrogen synthesis. As revealed by in vitro reporter gene assay and in vivo chromatin immunoprecipitation assay, AhR cooperates with an orphan nuclear receptor, Ad4BP/SF-1, to activate Cyp19 gene transcription in ovarian granulosa cells. Administration to female mice of an AhR ligand, DMBA (9,10-dimethyl-1,2-benzanthracene), induced ovarian Cyp19 gene expression, irrespective of the intrinsic phase of the estrus cycle. In addition to elucidating a physiological function for AhR, our studies also suggest a possible mechanism for the toxic effects of exogenous AhR ligands as endocrine disruptors.
Figures

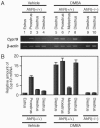
Similar articles
-
Regulation of aryl hydrocarbon receptor expression in rat granulosa cells.Biol Reprod. 2006 Sep;75(3):360-9. doi: 10.1095/biolreprod.106.053017. Epub 2006 May 31. Biol Reprod. 2006. PMID: 16738223
-
Modulation of oestrogen receptor signalling by association with the activated dioxin receptor.Nature. 2003 May 29;423(6939):545-50. doi: 10.1038/nature01606. Nature. 2003. PMID: 12774124
-
Disruption of aryl hydrocarbon receptor (AhR) induces regression of the seminal vesicle in aged male mice.Sex Dev. 2008;2(1):1-11. doi: 10.1159/000117714. Epub 2008 Apr 15. Sex Dev. 2008. PMID: 18418030
-
Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish.Gen Comp Endocrinol. 2008 Jan 1;155(1):31-62. doi: 10.1016/j.ygcen.2007.03.005. Epub 2007 Mar 21. Gen Comp Endocrinol. 2008. PMID: 17459383 Review.
-
Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction.Reproduction. 2005 Apr;129(4):379-89. doi: 10.1530/rep.1.00294. Reproduction. 2005. PMID: 15798013 Review.
Cited by
-
Methoxychlor inhibits growth and induces atresia through the aryl hydrocarbon receptor pathway in mouse ovarian antral follicles.Reprod Toxicol. 2012 Aug;34(1):16-21. doi: 10.1016/j.reprotox.2012.03.007. Epub 2012 Mar 30. Reprod Toxicol. 2012. PMID: 22484361 Free PMC article.
-
Testosterone-dependent interaction between androgen receptor and aryl hydrocarbon receptor induces liver receptor homolog 1 expression in rat granulosa cells.Mol Cell Biol. 2013 Aug;33(15):2817-28. doi: 10.1128/MCB.00011-13. Epub 2013 May 20. Mol Cell Biol. 2013. PMID: 23689136 Free PMC article.
-
The role of the aryl hydrocarbon receptor in the female reproductive system.Biochem Pharmacol. 2009 Feb 15;77(4):547-59. doi: 10.1016/j.bcp.2008.09.037. Epub 2008 Oct 14. Biochem Pharmacol. 2009. PMID: 18977336 Free PMC article. Review.
-
Combination effects of (tri)azole fungicides on hormone production and xenobiotic metabolism in a human placental cell line.Int J Environ Res Public Health. 2014 Sep 17;11(9):9660-79. doi: 10.3390/ijerph110909660. Int J Environ Res Public Health. 2014. PMID: 25233012 Free PMC article.
-
Estrogen receptor and aryl hydrocarbon receptor signaling pathways.Nucl Recept Signal. 2006;4:e016. doi: 10.1621/nrs.04016. Epub 2006 Jul 7. Nucl Recept Signal. 2006. PMID: 16862222 Free PMC article.
References
-
- Abbott, B. D., J. E. Schmid, J. A. Pitt, A. R. Buckalew, C. R. Wood, G. A. Held, and J. J. Diliberto. 1999. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol. Appl. Pharmacol. 155:62-70. - PubMed
-
- Baba, T., J. Mimura, K. Gradin, A. Kuroiwa, T. Watanabe, Y. Matsuda, J. Inazawa, K. Sogawa, and Y. Fujii-Kuriyama. 2001. Structure and expression of the Ah receptor repressor gene. J. Biol. Chem. 276:33101-33110. - PubMed
-
- Benedict, J. C., T. M. Lin, I. K. Loeffler, R. E. Peterson, and J. A. Flaws. 2000. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol. Sci. 56:382-388. - PubMed
-
- Benedict, J. C., K. P. Miller, T. M. Lin, C. Greenfeld, J. K. Babus, R. E. Peterson, and J. A. Flaws. 2003. Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol. Reprod. 68:1511-1517. - PubMed
-
- Botrugno, O. A., E. Fayard, J. S. Annicotte, C. Haby, T. Brennan, O. Wendling, T. Tanaka, T. Kodama, W. Thomas, J. Auwerx, and K. Schoonjans. 2004. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol. Cell 15:499-509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
