Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism
- PMID: 16300682
- PMCID: PMC1325233
- DOI: 10.1186/1743-7075-2-33
Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism
Abstract
Background: The metabolic function of PEPCK-C is not fully understood; deletion of the gene for the enzyme in mice provides an opportunity to fully assess its function.
Methods: The gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) (EC 4.1.1.32) (PEPCK-C) was deleted in mice by homologous recombination (PEPCK-C-/- mice) and the metabolic consequences assessed.
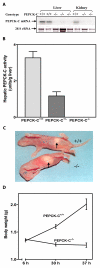
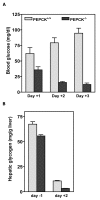
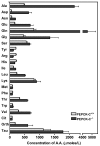
Results: PEPCK-C-/- mice became severely hypoglycemic by day two after birth and then died with profound hypoglycemia (12 mg/dl). The mice had milk in their stomachs at day two after birth and the administration of glucose raised the concentration of blood glucose in the mice but did not result in an increased survival. PEPCK-C-/- mice have two to three times the hepatic triglyceride content as control littermates on the second day after birth. These mice also had an elevation of lactate (2.5 times), beta-hydroxybutyrate (3 times) and triglyceride (50%) in their blood, as compared to control animals. On day two after birth, alanine, glycine, glutamine, glutamate, aspartate and asparagine were elevated in the blood of the PEPCK-C-/- mice and the blood urea nitrogen concentration was increased by 2-fold. The rate of oxidation of [2-14C]-acetate, and [5-14C]-glutamate to 14CO2 by liver slices from PEPCK-C-/- mice at two days of age was greatly reduced, as was the rate of fatty acid synthesis from acetate and glucose. As predicted by the lack of PEPCK-C, the concentration of malate in the livers of the PEPCK-C-/- mice was 10 times that of controls.
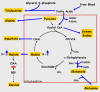
Conclusion: We conclude that PEPCK-C is required not only for gluconeogenesis and glyceroneogenesis but also for cataplerosis (i.e. the removal of citric acid cycle anions) and that the failure of this process in the livers of PEPCK-C-/- mice results in a marked reduction in citric acid cycle flux and the shunting of hepatic lipid into triglyceride, resulting in a fatty liver.
Figures

Similar articles
-
PCK1 and PCK2 as candidate diabetes and obesity genes.Cell Biochem Biophys. 2007;48(2-3):89-95. doi: 10.1007/s12013-007-0025-6. Cell Biochem Biophys. 2007. PMID: 17709878 Review.
-
Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase.J Biol Chem. 2004 Nov 19;279(47):48941-9. doi: 10.1074/jbc.M407120200. Epub 2004 Sep 3. J Biol Chem. 2004. PMID: 15347677
-
Cytosolic phosphoenolpyruvate carboxykinase as a cataplerotic pathway in the small intestine.Am J Physiol Gastrointest Liver Physiol. 2018 Aug 1;315(2):G249-G258. doi: 10.1152/ajpgi.00039.2018. Epub 2018 Apr 6. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29631378 Free PMC article.
-
Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver.Cell Metab. 2007 Apr;5(4):313-20. doi: 10.1016/j.cmet.2007.03.004. Cell Metab. 2007. PMID: 17403375 Free PMC article.
-
Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression.Annu Rev Biochem. 1997;66:581-611. doi: 10.1146/annurev.biochem.66.1.581. Annu Rev Biochem. 1997. PMID: 9242918 Review.
Cited by
-
Gluconeogenic enzyme PCK1 deficiency promotes CHK2 O-GlcNAcylation and hepatocellular carcinoma growth upon glucose deprivation.J Clin Invest. 2021 Apr 15;131(8):e144703. doi: 10.1172/JCI144703. J Clin Invest. 2021. PMID: 33690219 Free PMC article.
-
Small molecules _targeting selective PCK1 and PGC-1α lysine acetylation cause anti-diabetic action through increased lactate oxidation.Cell Chem Biol. 2024 Oct 17;31(10):1772-1786.e5. doi: 10.1016/j.chembiol.2024.09.001. Epub 2024 Sep 27. Cell Chem Biol. 2024. PMID: 39341205
-
AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver.J Biol Chem. 2008 Dec 5;283(49):33902-10. doi: 10.1074/jbc.M802537200. Epub 2008 Sep 17. J Biol Chem. 2008. PMID: 18801732 Free PMC article.
-
Gluconeogenesis in cancer cells - Repurposing of a starvation-induced metabolic pathway?Biochim Biophys Acta Rev Cancer. 2019 Aug;1872(1):24-36. doi: 10.1016/j.bbcan.2019.05.006. Epub 2019 May 30. Biochim Biophys Acta Rev Cancer. 2019. PMID: 31152822 Free PMC article. Review.
-
A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis.J Biol Chem. 2014 Mar 14;289(11):7257-63. doi: 10.1074/jbc.C113.544759. Epub 2014 Feb 4. J Biol Chem. 2014. PMID: 24497630 Free PMC article.
References
-
- Hanson RW, Patel YM. P-enolpyruvate carboxykinase: the gene and the enzyme. In: Meister A, editor. Advances in Enzymology. Vol. 69. New York, John Wiley and Sons; 1994. pp. 203–281. - PubMed
-
- Reshef L, Hanson RW, Ballard FJ. A possible physiological role for glyceroneogenesis in rat adipose tissue. J Biol Chem. 1970;245:5979–5984. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

