Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism
- PMID: 16300682
- PMCID: PMC1325233
- DOI: 10.1186/1743-7075-2-33
Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism
Abstract
Background: The metabolic function of PEPCK-C is not fully understood; deletion of the gene for the enzyme in mice provides an opportunity to fully assess its function.
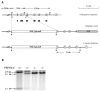
Methods: The gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) (EC 4.1.1.32) (PEPCK-C) was deleted in mice by homologous recombination (PEPCK-C-/- mice) and the metabolic consequences assessed.
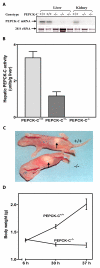
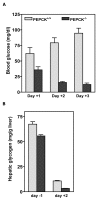
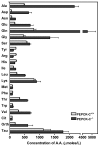
Results: PEPCK-C-/- mice became severely hypoglycemic by day two after birth and then died with profound hypoglycemia (12 mg/dl). The mice had milk in their stomachs at day two after birth and the administration of glucose raised the concentration of blood glucose in the mice but did not result in an increased survival. PEPCK-C-/- mice have two to three times the hepatic triglyceride content as control littermates on the second day after birth. These mice also had an elevation of lactate (2.5 times), beta-hydroxybutyrate (3 times) and triglyceride (50%) in their blood, as compared to control animals. On day two after birth, alanine, glycine, glutamine, glutamate, aspartate and asparagine were elevated in the blood of the PEPCK-C-/- mice and the blood urea nitrogen concentration was increased by 2-fold. The rate of oxidation of [2-14C]-acetate, and [5-14C]-glutamate to 14CO2 by liver slices from PEPCK-C-/- mice at two days of age was greatly reduced, as was the rate of fatty acid synthesis from acetate and glucose. As predicted by the lack of PEPCK-C, the concentration of malate in the livers of the PEPCK-C-/- mice was 10 times that of controls.
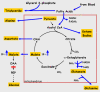
Conclusion: We conclude that PEPCK-C is required not only for gluconeogenesis and glyceroneogenesis but also for cataplerosis (i.e. the removal of citric acid cycle anions) and that the failure of this process in the livers of PEPCK-C-/- mice results in a marked reduction in citric acid cycle flux and the shunting of hepatic lipid into triglyceride, resulting in a fatty liver.
Figures

Similar articles
-
PCK1 and PCK2 as candidate diabetes and obesity genes.Cell Biochem Biophys. 2007;48(2-3):89-95. doi: 10.1007/s12013-007-0025-6. Cell Biochem Biophys. 2007. PMID: 17709878 Review.
-
Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase.J Biol Chem. 2004 Nov 19;279(47):48941-9. doi: 10.1074/jbc.M407120200. Epub 2004 Sep 3. J Biol Chem. 2004. PMID: 15347677
-
Cytosolic phosphoenolpyruvate carboxykinase as a cataplerotic pathway in the small intestine.Am J Physiol Gastrointest Liver Physiol. 2018 Aug 1;315(2):G249-G258. doi: 10.1152/ajpgi.00039.2018. Epub 2018 Apr 6. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29631378 Free PMC article.
-
Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver.Cell Metab. 2007 Apr;5(4):313-20. doi: 10.1016/j.cmet.2007.03.004. Cell Metab. 2007. PMID: 17403375 Free PMC article.
-
Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression.Annu Rev Biochem. 1997;66:581-611. doi: 10.1146/annurev.biochem.66.1.581. Annu Rev Biochem. 1997. PMID: 9242918 Review.
Cited by
-
Human Metabolic Enzymes Deficiency: A Genetic Mutation Based Approach.Scientifica (Cairo). 2016;2016:9828672. doi: 10.1155/2016/9828672. Epub 2016 Mar 9. Scientifica (Cairo). 2016. PMID: 27051561 Free PMC article. Review.
-
PEPCK-M expression in mouse liver potentiates, not replaces, PEPCK-C mediated gluconeogenesis.J Hepatol. 2013 Jul;59(1):105-13. doi: 10.1016/j.jhep.2013.02.020. Epub 2013 Mar 4. J Hepatol. 2013. PMID: 23466304 Free PMC article.
-
Flexibility and Adaptation of Cancer Cells in a Heterogenous Metabolic Microenvironment.Int J Mol Sci. 2021 Feb 2;22(3):1476. doi: 10.3390/ijms22031476. Int J Mol Sci. 2021. PMID: 33540663 Free PMC article. Review.
-
What is the metabolic role of phosphoenolpyruvate carboxykinase?J Biol Chem. 2009 Oct 2;284(40):27025-9. doi: 10.1074/jbc.R109.040543. Epub 2009 Jul 27. J Biol Chem. 2009. PMID: 19636077 Free PMC article. Review. No abstract available.
-
Liver Glucokinase(A456V) Induces Potent Hypoglycemia without Dyslipidemia through a Paradoxical Induction of the Catalytic Subunit of Glucose-6-Phosphatase.Int J Endocrinol. 2011;2011:707928. doi: 10.1155/2011/707928. Epub 2011 Dec 13. Int J Endocrinol. 2011. PMID: 22194744 Free PMC article.
References
-
- Hanson RW, Patel YM. P-enolpyruvate carboxykinase: the gene and the enzyme. In: Meister A, editor. Advances in Enzymology. Vol. 69. New York, John Wiley and Sons; 1994. pp. 203–281. - PubMed
-
- Reshef L, Hanson RW, Ballard FJ. A possible physiological role for glyceroneogenesis in rat adipose tissue. J Biol Chem. 1970;245:5979–5984. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

