Pathogenesis of osteoporosis: concepts, conflicts, and prospects
- PMID: 16322775
- PMCID: PMC1297264
- DOI: 10.1172/JCI27071
Pathogenesis of osteoporosis: concepts, conflicts, and prospects
Abstract
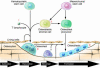
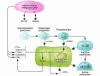
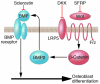
Osteoporosis is a disorder in which loss of bone strength leads to fragility fractures. This review examines the fundamental pathogenetic mechanisms underlying this disorder, which include: (a) failure to achieve a skeleton of optimal strength during growth and development; (b) excessive bone resorption resulting in loss of bone mass and disruption of architecture; and (c) failure to replace lost bone due to defects in bone formation. Estrogen deficiency is known to play a critical role in the development of osteoporosis, while calcium and vitamin D deficiencies and secondary hyperparathyroidism also contribute. There are multiple mechanisms underlying the regulation of bone remodeling, and these involve not only the osteoblastic and osteoclastic cell lineages but also other marrow cells, in addition to the interaction of systemic hormones, local cytokines, growth factors, and transcription factors. Polymorphisms of a large number of genes have been associated with differences in bone mass and fragility. It is now possible to diagnose osteoporosis, assess fracture risk, and reduce that risk with antiresorptive or other available therapies. However, new and more effective approaches are likely to emerge from a better understanding of the regulators of bone cell function.
Figures
Similar articles
-
Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology.J Mol Med (Berl). 2001 Jun;79(5-6):243-53. doi: 10.1007/s001090100226. J Mol Med (Berl). 2001. PMID: 11485016 Review.
-
The actions and interactions of sex steroids and growth factors/cytokines on the skeleton.Mol Endocrinol. 1999 Jun;13(6):819-28. doi: 10.1210/mend.13.6.0299. Mol Endocrinol. 1999. PMID: 10379881 Review. No abstract available.
-
Osteoporosis in hemodialysis patients revisited by bone histomorphometry: a new insight into an old problem.Kidney Int. 2006 May;69(10):1852-7. doi: 10.1038/sj.ki.5000311. Kidney Int. 2006. PMID: 16612334
-
The skeleton in primary hyperparathyroidism: a review focusing on bone remodeling, structure, mass, and fracture.APMIS Suppl. 2001;(102):1-52. APMIS Suppl. 2001. PMID: 11419022 Review.
-
Interaction between bone and immune cells: Implications for postmenopausal osteoporosis.Semin Cell Dev Biol. 2022 Mar;123:14-21. doi: 10.1016/j.semcdb.2021.05.014. Epub 2021 May 20. Semin Cell Dev Biol. 2022. PMID: 34024716 Review.
Cited by
-
Injectable double-crosslinked bone cement with enhanced bone adhesion and improved osteoporotic pathophysiological microenvironment for osteoregeneration in osteoporosis.Bioact Mater. 2024 Oct 2;43:441-459. doi: 10.1016/j.bioactmat.2024.09.032. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39399835 Free PMC article.
-
Screening of genes co-associated with osteoporosis and chronic HBV infection based on bioinformatics analysis and machine learning.Front Immunol. 2024 Sep 16;15:1472354. doi: 10.3389/fimmu.2024.1472354. eCollection 2024. Front Immunol. 2024. PMID: 39351238 Free PMC article.
-
Additional effects of herbal medicine combined with bisphosphonates for primary osteoporosis: a systematic review and meta-analysis.Front Pharmacol. 2024 Sep 13;15:1413515. doi: 10.3389/fphar.2024.1413515. eCollection 2024. Front Pharmacol. 2024. PMID: 39346562 Free PMC article.
-
Potential drug _targets for osteoporosis identified: A Mendelian randomization study.Heliyon. 2024 Aug 19;10(16):e36566. doi: 10.1016/j.heliyon.2024.e36566. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39253131 Free PMC article.
-
Sirt1: An Increasingly Interesting Molecule with a Potential Role in Bone Metabolism and Osteoporosis.Biomolecules. 2024 Aug 8;14(8):970. doi: 10.3390/biom14080970. Biomolecules. 2024. PMID: 39199358 Free PMC article. Review.
References
-
- Cooper, A., and Cooper, B.B. 1822. A treatise on dislocations, and on fractures of the joints. Churchill. London, United Kingdom. 425 pp. - PubMed
-
- Schapira D, Schapira C. Osteoporosis: the evolution of a scientific term. Osteoporos. Int. 1992;2:164–167. - PubMed
-
- Albright F, Bloomberg E, Smith PH. Postmenopausal osteoporosis. Trans. Assoc. Am. Physicians. 1940;55:298–305.
-
- Liu YZ, Liu YJ, Recker RR, Deng HW. Molecular studies of identification of genes for osteoporosis: the 2002 update. J. Endocrinol. 2003;177:147–196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

