Proteome analysis of Streptococcus thermophilus grown in milk reveals pyruvate formate-lyase as the major upregulated protein
- PMID: 16332852
- PMCID: PMC1317329
- DOI: 10.1128/AEM.71.12.8597-8605.2005
Proteome analysis of Streptococcus thermophilus grown in milk reveals pyruvate formate-lyase as the major upregulated protein
Abstract
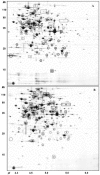
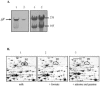
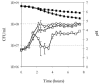
We investigated the adaptation to milk of Streptococcus thermophilus LMG18311 using a proteomic approach. Two-dimensional electrophoresis of cytosolic proteins were performed after growth in M17 medium or in milk. A major modification of the proteome concerned proteins involved in the supply of amino acids, like the peptidase PepX, and several enzymes involved in amino acid biosynthesis. In parallel, we observed the upregulation of the synthesis of seven enzymes directly involved in the synthesis of purines, as well as formyl-tetrahydrofolate (THF) synthetase and serine hydroxy-methyl transferase, two enzymes responsible for the synthesis of compounds (THF and glycine, respectively) feeding the purine biosynthetic pathway. The analysis also revealed a massive increase in the synthesis of pyruvate formate-lyase (PFL), the enzyme which converts pyruvate into acetyl coenzyme A and formate. PFL has been essentially studied for its role in mixed-acid product formation in lactic acid bacteria during anaerobic fermentation. However, formate is an important methyl group donor for anabolic pathway through the formation of folate derivates. We hypothesized that PFL was involved in purine biosynthesis during growth in milk. We showed that PFL expression was regulated at the transcriptional level and that pfl transcription occurred during the exponential growth phase in milk. The complementation of milk with formate or purine bases was shown to reduce pfl expression, to suppress PFL synthesis, and to stimulate growth of S. thermophilus. These results show a novel regulatory mechanism controlling the synthesis of PFL and suggest an unrecognized physiological role for PFL as a formate supplier for anabolic purposes.
Figures


Similar articles
-
Molecular characteristics and transcription of the gene encoding a multifunctional alcohol dehydrogenase in relation to the deactivation of pyruvate formate-lyase in the ruminal bacterium Streptococcus bovis.Arch Microbiol. 2004 Feb;181(2):122-8. doi: 10.1007/s00203-003-0638-0. Epub 2003 Dec 16. Arch Microbiol. 2004. PMID: 14676990
-
Physiology of Streptococcus thermophilus during the late stage of milk fermentation with special regard to sulfur amino-acid metabolism.Proteomics. 2008 Oct;8(20):4273-86. doi: 10.1002/pmic.200700489. Proteomics. 2008. PMID: 18814336
-
A widespread picture of the Streptococcus thermophilus proteome by cell lysate fractionation and gel-based/gel-free approaches.Proteomics. 2007 May;7(9):1420-33. doi: 10.1002/pmic.200601030. Proteomics. 2007. PMID: 17407180
-
Differential proteomic analysis in the study of prokaryotes stress resistance.Ann Ist Super Sanita. 2005;41(4):459-68. Ann Ist Super Sanita. 2005. PMID: 16569914 Review.
-
A glycyl radical solution: oxygen-dependent interconversion of pyruvate formate-lyase.Mol Microbiol. 1998 Aug;29(4):945-54. doi: 10.1046/j.1365-2958.1998.00941.x. Mol Microbiol. 1998. PMID: 9767563 Review.
Cited by
-
Comparative Transcriptomic Analysis of Streptococcus thermophilus TH1436 and TH1477 Showing Different Capability in the Use of Galactose.Front Microbiol. 2018 Aug 7;9:1765. doi: 10.3389/fmicb.2018.01765. eCollection 2018. Front Microbiol. 2018. PMID: 30131781 Free PMC article.
-
Comparative mRNA-Seq Analysis Reveals the Improved EPS Production Machinery in Streptococcus thermophilus ASCC 1275 During Optimized Milk Fermentation.Front Microbiol. 2018 Mar 13;9:445. doi: 10.3389/fmicb.2018.00445. eCollection 2018. Front Microbiol. 2018. PMID: 29593689 Free PMC article.
-
An ancient riboswitch class in bacteria regulates purine biosynthesis and one-carbon metabolism.Mol Cell. 2015 Jan 22;57(2):317-28. doi: 10.1016/j.molcel.2015.01.001. Mol Cell. 2015. PMID: 25616067 Free PMC article.
-
Pyruvate formate lyase acts as a formate supplier for metabolic processes during anaerobiosis in Staphylococcus aureus.J Bacteriol. 2011 Feb;193(4):952-62. doi: 10.1128/JB.01161-10. Epub 2010 Dec 17. J Bacteriol. 2011. PMID: 21169491 Free PMC article.
-
Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea, and their metagenomes.Genome Biol. 2010;11(3):R31. doi: 10.1186/gb-2010-11-3-r31. Epub 2010 Mar 15. Genome Biol. 2010. PMID: 20230605 Free PMC article.
References
-
- Anastasiou, R., M. Papadelli, M. D. Georgalaki, G. Kalantzopoulos, and E. Tsakalidou. 2002. Cloning and sequencing of the gene encoding X-prolyl-dipeptidyl aminopeptidase (PepX) from Streptococcus thermophilus strain ACA-DC 4. J. Appl. Microbiol. 93:52-59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

