CCR2 modulates inflammatory and metabolic effects of high-fat feeding
- PMID: 16341265
- PMCID: PMC1307559
- DOI: 10.1172/JCI24335
CCR2 modulates inflammatory and metabolic effects of high-fat feeding
Erratum in
- J Clin Invest. 2006 May;116(5):1457
Abstract
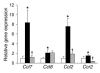
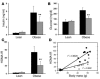
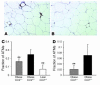
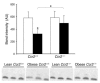
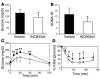
The C-C motif chemokine receptor-2 (CCR2) regulates monocyte and macrophage recruitment and is necessary for macrophage-dependent inflammatory responses and the development of atherosclerosis. Although adipose tissue expression and circulating concentrations of CCL2 (also known as MCP1), a high-affinity ligand for CCR2, are elevated in obesity, the role of CCR2 in metabolic disorders, including insulin resistance, hepatic steatosis, and inflammation associated with obesity, has not been studied. To determine what role CCR2 plays in the development of metabolic phenotypes, we studied the effects of Ccr2 genotype on the development of obesity and its associated phenotypes. Genetic deficiency in Ccr2 reduced food intake and attenuated the development of obesity in mice fed a high-fat diet. In obese mice matched for adiposity, Ccr2 deficiency reduced macrophage content and the inflammatory profile of adipose tissue, increased adiponectin expression, ameliorated hepatic steatosis, and improved systemic glucose homeostasis and insulin sensitivity. In mice with established obesity, short-term treatment with a pharmacological antagonist of CCR2 lowered macrophage content of adipose tissue and improved insulin sensitivity without significantly altering body mass or improving hepatic steatosis. These data suggest that CCR2 influences the development of obesity and associated adipose tissue inflammation and systemic insulin resistance and plays a role in the maintenance of adipose tissue macrophages and insulin resistance once obesity and its metabolic consequences are established.
Figures

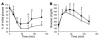
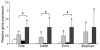
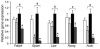
Comment in
-
Inflamed fat: what starts the fire?J Clin Invest. 2006 Jan;116(1):33-5. doi: 10.1172/JCI27280. J Clin Invest. 2006. PMID: 16395402 Free PMC article.
Similar articles
-
Inflamed fat: what starts the fire?J Clin Invest. 2006 Jan;116(1):33-5. doi: 10.1172/JCI27280. J Clin Invest. 2006. PMID: 16395402 Free PMC article.
-
An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice.Endocrinology. 2010 Mar;151(3):971-9. doi: 10.1210/en.2009-0926. Epub 2010 Jan 7. Endocrinology. 2010. PMID: 20056828
-
[Macrophages, inflammation, adipose tissue, obesity and insulin resistance].Gac Med Mex. 2007 Nov-Dec;143(6):505-12. Gac Med Mex. 2007. PMID: 18269082 Review. Spanish.
-
Diet induction of monocyte chemoattractant protein-1 and its impact on obesity.Obes Res. 2005 Aug;13(8):1311-20. doi: 10.1038/oby.2005.159. Obes Res. 2005. PMID: 16129712
-
Does C-C Motif Chemokine Ligand 2 (CCL2) Link Obesity to a Pro-Inflammatory State?Int J Mol Sci. 2021 Feb 2;22(3):1500. doi: 10.3390/ijms22031500. Int J Mol Sci. 2021. PMID: 33540898 Free PMC article. Review.
Cited by
-
Adipokines: masterminds of metabolic inflammation.Nat Rev Immunol. 2024 Nov 7. doi: 10.1038/s41577-024-01103-8. Online ahead of print. Nat Rev Immunol. 2024. PMID: 39511425 Review.
-
Exacerbation of atherosclerosis, hyperlipidemia and inflammation by MK886, an inhibitor of leukotriene biosynthesis, in obese and diabetic mice.Curr Res Pharmacol Drug Discov. 2024 Oct 10;7:100203. doi: 10.1016/j.crphar.2024.100203. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 39497763 Free PMC article.
-
Obesity-induced fibrosis in osteoarthritis: Pathogenesis, consequences and novel therapeutic opportunities.Osteoarthr Cartil Open. 2024 Aug 17;6(4):100511. doi: 10.1016/j.ocarto.2024.100511. eCollection 2024 Dec. Osteoarthr Cartil Open. 2024. PMID: 39483440 Free PMC article. Review.
-
A Missing Puzzle in Preclinical Studies-Are CCR2, CCR5, and Their Ligands' Roles Similar in Obesity-Induced Hypersensitivity and Diabetic Neuropathy?-Evidence from Rodent Models and Clinical Studies.Int J Mol Sci. 2024 Oct 21;25(20):11323. doi: 10.3390/ijms252011323. Int J Mol Sci. 2024. PMID: 39457105 Free PMC article. Review.
-
Unravelling monocyte functions: from the guardians of health to the regulators of disease.Discov Immunol. 2024 Aug 30;3(1):kyae014. doi: 10.1093/discim/kyae014. eCollection 2024. Discov Immunol. 2024. PMID: 39430099 Free PMC article. Review.
References
-
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005;96:939–949. - PubMed
-
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. - PubMed
-
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

