XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands
- PMID: 16362047
- PMCID: PMC1356340
- DOI: 10.1038/sj.emboj.7600903
XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands
Abstract
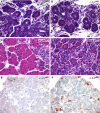
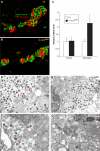
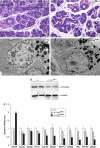
The secretory function of cells relies on the capacity of the endoplasmic reticulum (ER) to fold and modify nascent polypeptides and to synthesize phospholipids for the subsequent trafficking of secretory proteins through the ER-Golgi network. We have previously demonstrated that the transcription factor XBP-1 activates the expression of certain ER chaperone genes and initiates ER biogenesis. Here, we have rescued the embryonic lethality of XBP-1 deficient fetuses by _targeting an XBP-1 transgene selectively to hepatocytes (XBP-1-/-;LivXBP1). XBP-1-/-;LivXBP1 mice displayed abnormalities exclusively in secretory organs such as exocrine pancreas and salivary gland that led to early postnatal lethality from impaired production of pancreatic digestive enzymes. The ER was poorly developed in pancreatic and salivary gland acinar cells, accompanied by decreased expression of ER chaperone genes. Marked apoptosis of pancreatic acinar cells was observed during embryogenesis. Thus, the absence of XBP-1 results in an imbalance between the cargo load on the ER and its capacity to handle it, leading to the activation of ER stress-mediated proapoptotic pathways. These data lead us to propose that XBP-1 is both necessary and sufficient for the full biogenesis of the secretory machinery in exocrine cells.
Figures
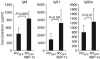
Similar articles
-
Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis.J Biol Chem. 2007 Mar 9;282(10):7024-34. doi: 10.1074/jbc.M609490200. Epub 2007 Jan 8. J Biol Chem. 2007. PMID: 17213183
-
XBP-1, a key regulator of unfolded protein response, activates transcription of IGF1 and Akt phosphorylation in zebrafish embryonic cell line.Biochem Biophys Res Commun. 2007 Aug 3;359(3):778-83. doi: 10.1016/j.bbrc.2007.05.183. Epub 2007 Jun 4. Biochem Biophys Res Commun. 2007. PMID: 17560942
-
Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis.Proc Natl Acad Sci U S A. 2008 Jan 15;105(2):757-62. doi: 10.1073/pnas.0711094105. Epub 2008 Jan 4. Proc Natl Acad Sci U S A. 2008. PMID: 18178615 Free PMC article.
-
An overview of pancreatic exocrine secretion.Comp Biochem Physiol B. 1984;78(1):1-13. doi: 10.1016/0305-0491(84)90136-6. Comp Biochem Physiol B. 1984. PMID: 6378509 Review.
-
The endocrine secretion of mammalian digestive enzymes by exocrine glands.Am J Physiol. 1999 Feb;276(2):E223-32. doi: 10.1152/ajpendo.1999.276.2.E223. Am J Physiol. 1999. PMID: 9950780 Review.
Cited by
-
Conditional Loss of Nmp4 in Mesenchymal Stem Progenitor Cells Enhances PTH-Induced Bone Formation.J Bone Miner Res. 2023 Jan;38(1):70-85. doi: 10.1002/jbmr.4732. Epub 2022 Nov 22. J Bone Miner Res. 2023. PMID: 36321253 Free PMC article.
-
XBP1S associates with RUNX2 and regulates chondrocyte hypertrophy.J Biol Chem. 2012 Oct 5;287(41):34500-13. doi: 10.1074/jbc.M112.385922. Epub 2012 Aug 3. J Biol Chem. 2012. Retraction in: J Biol Chem. 2015 Apr 24;290(17):10643. doi: 10.1074/jbc.A112.385922 PMID: 22865880 Free PMC article. Retracted.
-
Lysine acetylation in the lumen of the ER: a novel and essential function under the control of the UPR.Biochim Biophys Acta. 2013 Mar;1833(3):686-97. doi: 10.1016/j.bbamcr.2012.12.004. Epub 2012 Dec 13. Biochim Biophys Acta. 2013. PMID: 23247107 Free PMC article. Review.
-
The specialized unfolded protein response of B lymphocytes: ATF6α-independent development of antibody-secreting B cells.Mol Immunol. 2012 Jul;51(3-4):347-55. doi: 10.1016/j.molimm.2012.04.001. Epub 2012 May 1. Mol Immunol. 2012. PMID: 22555069 Free PMC article.
-
Nodal signaling activates differentiation genes during zebrafish gastrulation.Dev Biol. 2007 Apr 15;304(2):525-40. doi: 10.1016/j.ydbio.2007.01.012. Epub 2007 Jan 12. Dev Biol. 2007. PMID: 17306247 Free PMC article.
References
-
- Aridor M, Balch WE (1999) Integration of endoplasmic reticulum signaling in health and disease. Nat Med 5: 745–751 - PubMed
-
- Beaudoin AR, Grondin G (1991) Secretory pathways in animal cells: with emphasis on pancreatic acinar cells. J Electron Microsc Tech 17: 51–69 - PubMed
-
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332 - PubMed
-
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96 - PubMed
-
- Castle JD, Castle AM (1993) Sorting and secretion of salivary proteins. Crit Rev Oral Biol Med 4: 393–398 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

