Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells
- PMID: 16418332
- PMCID: PMC1895278
- DOI: 10.1182/blood-2005-08-3434
Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells
Abstract
Perifosine is a synthetic novel alkylphospholipid, a new class of antitumor agents which _targets cell membranes and inhibits Akt activation. Here we show that baseline phosphorylation of Akt in multiple myeloma (MM) cells is completely inhibited by perifosine [octadecyl-(1,1-dimethyl-piperidinio-4-yl)-phosphate] in a time- and dose-dependent fashion, without inhibiting phosphoinositide-dependent protein kinase 1 phosphorylation. Perifosine induces significant cytotoxicity in both MM cell lines and patient MM cells resistant to conventional therapeutic agents. Perifosine does not induce cytotoxicity in peripheral blood mononuclear cells. Neither exogenous interleukin-6 (IL-6) nor insulinlike growth factor 1 (IGF-1) overcomes Perifosine-induced cytotoxicity. Importantly, Perifosine induces apoptosis even of MM cells adherent to bone marrow stromal cells. Perifosine triggers c-Jun N-terminal kinase (JNK) activation, followed by caspase-8/9 and poly (ADP)-ribose polymerase cleavage. Inhibition of JNK abrogates perifosine-induced cytotoxicity, suggesting that JNK plays an essential role in perifosine-induced apoptosis. Interestingly, phosphorylation of extracellular signal-related kinase (ERK) is increased by perifosine; conversely, MEK inhibitor synergistically enhances Perifosine-induced cytotoxicity in MM cells. Furthermore, perifosine augments dexamethasone, doxorubicin, melphalan, and bortezomib-induced MM cell cytotoxicity. Finally, perifosine demonstrates significant antitumor activity in a human plasmacytoma mouse model, associated with down-regulation of Akt phosphorylation in tumor cells. Taken together, our data provide the rationale for clinical trials of perifosine to improve patient outcome in MM.
Figures


 ), Z-LEHD-FMK (
), Z-LEHD-FMK ( ), or Z-VAD-FMK (▪). (D) MM.1S cells were cultured with perifosine (10 μM) for the indicated periods. Cells were then lysed and subjected to Western blotting using anti–p-p38 MAPK, anti-p38 MAPK, anti–p-JNK, anti-JNK, and α-tubulin Abs. (E) MM.1S cells were cultured with perifosine (5 μM and 10 μM) for 8 hours, in the presence or absence of SP600125 (10 μM). Cells were then lysed and subjected to Western blotting using anti–p-JNK, anti-JNK, and caspase-8 Abs. (F) MM.1S cells were cultured for 24 hours with control media (□), or with 2.5 μM (
), or Z-VAD-FMK (▪). (D) MM.1S cells were cultured with perifosine (10 μM) for the indicated periods. Cells were then lysed and subjected to Western blotting using anti–p-p38 MAPK, anti-p38 MAPK, anti–p-JNK, anti-JNK, and α-tubulin Abs. (E) MM.1S cells were cultured with perifosine (5 μM and 10 μM) for 8 hours, in the presence or absence of SP600125 (10 μM). Cells were then lysed and subjected to Western blotting using anti–p-JNK, anti-JNK, and caspase-8 Abs. (F) MM.1S cells were cultured for 24 hours with control media (□), or with 2.5 μM ( ), 5 μM (
), 5 μM ( ), and 7.5 μM(▪) perifosine, in the presence or absence of SP600126 (10 μM). (G) MM.1S cells were transiently transfected with control (•) or JNK2 siRNA expression plasmid (▪). Whole-cell lysates were subjected to Western blotting using anti-JNK2 and α-tubulin Abs. (H) MM.1S cells were cultured for 24 hours with control media (□) or perifosine (5 and 7.5 μM) in the presence or absence of control media (□), 200 nM (
), and 7.5 μM(▪) perifosine, in the presence or absence of SP600126 (10 μM). (G) MM.1S cells were transiently transfected with control (•) or JNK2 siRNA expression plasmid (▪). Whole-cell lysates were subjected to Western blotting using anti-JNK2 and α-tubulin Abs. (H) MM.1S cells were cultured for 24 hours with control media (□) or perifosine (5 and 7.5 μM) in the presence or absence of control media (□), 200 nM ( ), or 400 nM (▪) SCIO469. Cytotoxicity was assessed by MTT assay; data represent mean (± SD) of quadruplicate cultures.
), or 400 nM (▪) SCIO469. Cytotoxicity was assessed by MTT assay; data represent mean (± SD) of quadruplicate cultures.

 ) or 10 μM(▪) perifosine in the presence or absence of U0126 (5 μM). Cytotoxicity was assessed by MTT assay; data represent means (± SD) of quadruplicate cultures. (F) MM.1S cells were cultured for 48 hours with control media (□) and with 1.25 μM (light orange bars), 2.5 μM (medium orange bars), or 5 μM (dark orange bars) perifosine with or without U0126 (2.5 μM) and in the presence or absence of BMSCs. Cell proliferation was assessed by [3H]-thymidine uptake; data represent means (± SD) of quadruplicate cultures.
) or 10 μM(▪) perifosine in the presence or absence of U0126 (5 μM). Cytotoxicity was assessed by MTT assay; data represent means (± SD) of quadruplicate cultures. (F) MM.1S cells were cultured for 48 hours with control media (□) and with 1.25 μM (light orange bars), 2.5 μM (medium orange bars), or 5 μM (dark orange bars) perifosine with or without U0126 (2.5 μM) and in the presence or absence of BMSCs. Cell proliferation was assessed by [3H]-thymidine uptake; data represent means (± SD) of quadruplicate cultures.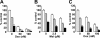
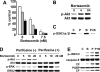
 ) or 5 μM (▪) perifosine in the presence or absence of bortezomib (5 nM and 7.5 nM). Cytotoxicity was assessed by MTT assay; data represent means (± SD) of quadruplicate cultures. (B) MM.1S cells were cultured with bortezomib (10 nM) for 4 hours and 8 hours. Whole-cell lysates were subjected to Western blotting with p-Akt and Akt Abs. (C) MM.1S cells were cultured with bortezomib (10 nM) for 8 hours. Whole-cell lysates were immunoprecipitated with anti-Akt Ab. The immunoprecipitates were washed and subjected to in vitro kinase assay, according to manufacturer's protocol. The reaction mixtures were immunoblotted with anti–p-GSK3α/β. (D) MM.1S cells were cultured with bortezomib (5, 10, and 20 nM) in the presence (5 μM) or absence of perifosine. Whole-cell lysates were subjected to Western blotting with p-Akt, Akt, p-ERK, and ERK2 Abs. (E) MM.1S cells were cultured for 8 hours with control media, perifosine (5 μM), bortezomib (10 nM), or perifosine (5 μM) plus Bortezomib (10 nM). Cells were then lysed and subjected to Western blotting using anti–p-JNK1/2, caspase-8, and PARP Abs.
) or 5 μM (▪) perifosine in the presence or absence of bortezomib (5 nM and 7.5 nM). Cytotoxicity was assessed by MTT assay; data represent means (± SD) of quadruplicate cultures. (B) MM.1S cells were cultured with bortezomib (10 nM) for 4 hours and 8 hours. Whole-cell lysates were subjected to Western blotting with p-Akt and Akt Abs. (C) MM.1S cells were cultured with bortezomib (10 nM) for 8 hours. Whole-cell lysates were immunoprecipitated with anti-Akt Ab. The immunoprecipitates were washed and subjected to in vitro kinase assay, according to manufacturer's protocol. The reaction mixtures were immunoblotted with anti–p-GSK3α/β. (D) MM.1S cells were cultured with bortezomib (5, 10, and 20 nM) in the presence (5 μM) or absence of perifosine. Whole-cell lysates were subjected to Western blotting with p-Akt, Akt, p-ERK, and ERK2 Abs. (E) MM.1S cells were cultured for 8 hours with control media, perifosine (5 μM), bortezomib (10 nM), or perifosine (5 μM) plus Bortezomib (10 nM). Cells were then lysed and subjected to Western blotting using anti–p-JNK1/2, caspase-8, and PARP Abs.
Similar articles
-
Dual inhibition of akt/mammalian _target of rapamycin pathway by nanoparticle albumin-bound-rapamycin and perifosine induces antitumor activity in multiple myeloma.Mol Cancer Ther. 2010 Apr;9(4):963-75. doi: 10.1158/1535-7163.MCT-09-0763. Epub 2010 Apr 6. Mol Cancer Ther. 2010. PMID: 20371718 Free PMC article.
-
Inhibition of Akt induces significant downregulation of survivin and cytotoxicity in human multiple myeloma cells.Br J Haematol. 2007 Sep;138(6):783-91. doi: 10.1111/j.1365-2141.2007.06714.x. Br J Haematol. 2007. PMID: 17760810
-
Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation.Mol Cancer Ther. 2003 Nov;2(11):1093-103. Mol Cancer Ther. 2003. PMID: 14617782
-
In vitro cytotoxicity of the novel antimyeloma agents perifosine, bortezomib and lenalidomide against different cell lines.Invest New Drugs. 2012 Apr;30(2):480-9. doi: 10.1007/s10637-010-9576-2. Epub 2010 Nov 16. Invest New Drugs. 2012. PMID: 21080211
-
Perifosine: update on a novel Akt inhibitor.Curr Oncol Rep. 2009 Mar;11(2):102-10. doi: 10.1007/s11912-009-0016-4. Curr Oncol Rep. 2009. PMID: 19216841 Free PMC article. Review.
Cited by
-
ZHX2 mediates proteasome inhibitor resistance via regulating nuclear translocation of NF-κB in multiple myeloma.Cancer Med. 2020 Oct;9(19):7244-7252. doi: 10.1002/cam4.3347. Epub 2020 Aug 11. Cancer Med. 2020. PMID: 32780537 Free PMC article.
-
Advances in the treatment of monoclonal gammopaties: The emerging role of _targeted therapy in plasma cell dyscrasias.Biologics. 2008 Sep;2(3):419-31. doi: 10.2147/btt.s3088. Biologics. 2008. PMID: 19707373 Free PMC article.
-
Role of Human Epicardial Adipose Tissue-Derived miR-92a-3p in Myocardial Redox State.J Am Coll Cardiol. 2023 Jul 25;82(4):317-332. doi: 10.1016/j.jacc.2023.05.031. J Am Coll Cardiol. 2023. PMID: 37468187 Free PMC article.
-
Anti-tumor activity of the pan-RAF inhibitor TAK-580 in combination with KPT-330 (selinexor) in multiple myeloma.Int J Hematol. 2022 Feb;115(2):233-243. doi: 10.1007/s12185-021-03244-1. Epub 2021 Nov 5. Int J Hematol. 2022. PMID: 34741230
-
_targeting NF-kappaB in Waldenstrom macroglobulinemia.Blood. 2008 May 15;111(10):5068-77. doi: 10.1182/blood-2007-09-115170. Epub 2008 Mar 11. Blood. 2008. PMID: 18334673 Free PMC article.
References
-
- Gregory WM, Richards MA, Malpas JS. Combination chemotherapy versus melphalan and prednisolone in the treatment of multiple myeloma: an overview of published trials. J Clin Oncol. 1992;10: 334-342. - PubMed
-
- Myeloma Trialists' Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. J Clin Oncol. 1998;16: 3832-3842. - PubMed
-
- Lenhoff S, Hjorth M, Holmberg E, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Blood. 2000;95: 7-11. - PubMed
-
- Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003; 349: 2495-2502. - PubMed
-
- Sonneveld P. Drug resistance in myeloma. Pathol Biol (Paris). 1999;47: 182-187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

