Dimerization and opposite base-dependent catalytic impairment of polymorphic S326C OGG1 glycosylase
- PMID: 16549874
- PMCID: PMC1405821
- DOI: 10.1093/nar/gkl060
Dimerization and opposite base-dependent catalytic impairment of polymorphic S326C OGG1 glycosylase
Abstract
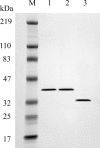
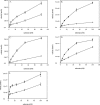
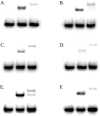
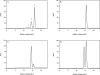
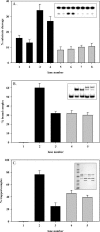
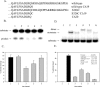
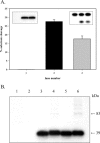
Human 8-oxoguanine-DNA glycosylase (OGG1) is the major enzyme for repairing 8-oxoguanine (8-oxoG), a mutagenic guanine base lesion produced by reactive oxygen species (ROS). A frequently occurring OGG1 polymorphism in human populations results in the substitution of serine 326 for cysteine (S326C). The 326 C/C genotype is linked to numerous cancers, although the mechanism of carcinogenesis associated with the variant is unclear. We performed detailed enzymatic studies of polymorphic OGG1 and found functional defects in the enzyme. S326C OGG1 excised 8-oxoG from duplex DNA and cleaved abasic sites at rates 2- to 6-fold lower than the wild-type enzyme, depending upon the base opposite the lesion. Binding experiments showed that the polymorphic OGG1 binds DNA damage with significantly less affinity than the wild-type enzyme. Remarkably, gel shift, chemical cross-linking and gel filtration experiments showed that S326C both exists in solution and binds damaged DNA as a dimer. S326C OGG1 enzyme expressed in human cells was also found to have reduced activity and a dimeric conformation. The glycosylase activity of S326C OGG1 was not significantly stimulated by the presence of AP-endonuclease. The altered substrate specificity, lack of stimulation by AP-endonuclease 1 (APE1) and anomalous DNA binding conformation of S326C OGG1 may contribute to its linkage to cancer incidence.
Figures

Similar articles
-
Mechanism of interaction between human 8-oxoguanine-DNA glycosylase and AP endonuclease.DNA Repair (Amst). 2007 Mar 1;6(3):317-28. doi: 10.1016/j.dnarep.2006.10.022. Epub 2006 Nov 27. DNA Repair (Amst). 2007. PMID: 17126083
-
Stimulation of human 8-oxoguanine-DNA glycosylase by AP-endonuclease: potential coordination of the initial steps in base excision repair.Nucleic Acids Res. 2001 Jan 15;29(2):430-8. doi: 10.1093/nar/29.2.430. Nucleic Acids Res. 2001. PMID: 11139613 Free PMC article.
-
Specificity of stimulation of human 8-oxoguanine-DNA glycosylase by AP endonuclease.Biochem Biophys Res Commun. 2008 Mar 28;368(1):175-9. doi: 10.1016/j.bbrc.2008.01.076. Epub 2008 Jan 24. Biochem Biophys Res Commun. 2008. PMID: 18222119
-
Multiple DNA glycosylases for repair of 8-oxoguanine and their potential in vivo functions.Prog Nucleic Acid Res Mol Biol. 2001;68:193-205. doi: 10.1016/s0079-6603(01)68100-5. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11554297 Review.
-
The OGG1 gene encodes a repair enzyme for oxidatively damaged DNA and is involved in human carcinogenesis.Antioxid Redox Signal. 2001 Aug;3(4):597-609. doi: 10.1089/15230860152542952. Antioxid Redox Signal. 2001. PMID: 11554447 Review.
Cited by
-
The Role of 8-oxoG Repair Systems in Tumorigenesis and Cancer Therapy.Cells. 2022 Nov 27;11(23):3798. doi: 10.3390/cells11233798. Cells. 2022. PMID: 36497058 Free PMC article. Review.
-
A quantitative PCR-based assay reveals that nucleotide excision repair plays a predominant role in the removal of DNA-protein crosslinks from plasmids transfected into mammalian cells.DNA Repair (Amst). 2018 Feb;62:18-27. doi: 10.1016/j.dnarep.2018.01.004. Epub 2018 Jan 9. DNA Repair (Amst). 2018. PMID: 29413806 Free PMC article.
-
RAS transformation requires CUX1-dependent repair of oxidative DNA damage.PLoS Biol. 2014 Mar 11;12(3):e1001807. doi: 10.1371/journal.pbio.1001807. eCollection 2014 Mar. PLoS Biol. 2014. PMID: 24618719 Free PMC article.
-
Involvement of oxidatively damaged DNA and repair in cancer development and aging.Am J Transl Res. 2010 May 15;2(3):254-84. Am J Transl Res. 2010. PMID: 20589166 Free PMC article.
-
MUTYH Tyr165Cys, OGG1 Ser326Cys and XPD Lys751Gln polymorphisms and head neck cancer susceptibility: a case control study.Mol Biol Rep. 2011 Feb;38(2):1251-61. doi: 10.1007/s11033-010-0224-x. Epub 2010 Jun 23. Mol Biol Rep. 2011. PMID: 20571908
References
-
- Doetsch P.W., Zasatawny T.H., Martin A.M., Dizdaroglu M. Monomeric base damage products from adenine, guanine, and thymine induced by exposure of DNA to ultraviolet radiation. Biochemistry. 1995;34:737–742. - PubMed
-
- Dizdaroglu M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry. 1985;24:4476–4481. - PubMed
-
- Kasai H., Nishimura S. Formation of 8-hydroxyguanosine in DNA by oxygen radicals and its biological significance. In: Seis H., editor. Oxidative Stress: Oxidants and Antioxidants. London: Academic Press; 1991. pp. 99–116.
-
- Cadet J., Berger M., Douki T., Ravanat J.L. Oxidative damage to DNA: formation, measurement, and biological significance. Rev. Physiol. Biochem. Pharmacol. 1997;131:1–87. - PubMed
-
- Barnes D.E., Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu. Rev. Genet. 2004;38:445–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

