Dimerization and opposite base-dependent catalytic impairment of polymorphic S326C OGG1 glycosylase
- PMID: 16549874
- PMCID: PMC1405821
- DOI: 10.1093/nar/gkl060
Dimerization and opposite base-dependent catalytic impairment of polymorphic S326C OGG1 glycosylase
Abstract
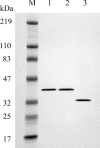
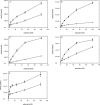
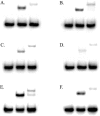
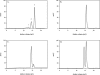
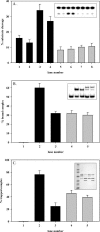
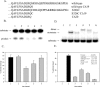
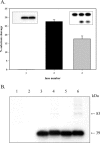
Human 8-oxoguanine-DNA glycosylase (OGG1) is the major enzyme for repairing 8-oxoguanine (8-oxoG), a mutagenic guanine base lesion produced by reactive oxygen species (ROS). A frequently occurring OGG1 polymorphism in human populations results in the substitution of serine 326 for cysteine (S326C). The 326 C/C genotype is linked to numerous cancers, although the mechanism of carcinogenesis associated with the variant is unclear. We performed detailed enzymatic studies of polymorphic OGG1 and found functional defects in the enzyme. S326C OGG1 excised 8-oxoG from duplex DNA and cleaved abasic sites at rates 2- to 6-fold lower than the wild-type enzyme, depending upon the base opposite the lesion. Binding experiments showed that the polymorphic OGG1 binds DNA damage with significantly less affinity than the wild-type enzyme. Remarkably, gel shift, chemical cross-linking and gel filtration experiments showed that S326C both exists in solution and binds damaged DNA as a dimer. S326C OGG1 enzyme expressed in human cells was also found to have reduced activity and a dimeric conformation. The glycosylase activity of S326C OGG1 was not significantly stimulated by the presence of AP-endonuclease. The altered substrate specificity, lack of stimulation by AP-endonuclease 1 (APE1) and anomalous DNA binding conformation of S326C OGG1 may contribute to its linkage to cancer incidence.
Figures

Similar articles
-
Mechanism of interaction between human 8-oxoguanine-DNA glycosylase and AP endonuclease.DNA Repair (Amst). 2007 Mar 1;6(3):317-28. doi: 10.1016/j.dnarep.2006.10.022. Epub 2006 Nov 27. DNA Repair (Amst). 2007. PMID: 17126083
-
Stimulation of human 8-oxoguanine-DNA glycosylase by AP-endonuclease: potential coordination of the initial steps in base excision repair.Nucleic Acids Res. 2001 Jan 15;29(2):430-8. doi: 10.1093/nar/29.2.430. Nucleic Acids Res. 2001. PMID: 11139613 Free PMC article.
-
Specificity of stimulation of human 8-oxoguanine-DNA glycosylase by AP endonuclease.Biochem Biophys Res Commun. 2008 Mar 28;368(1):175-9. doi: 10.1016/j.bbrc.2008.01.076. Epub 2008 Jan 24. Biochem Biophys Res Commun. 2008. PMID: 18222119
-
Multiple DNA glycosylases for repair of 8-oxoguanine and their potential in vivo functions.Prog Nucleic Acid Res Mol Biol. 2001;68:193-205. doi: 10.1016/s0079-6603(01)68100-5. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11554297 Review.
-
The OGG1 gene encodes a repair enzyme for oxidatively damaged DNA and is involved in human carcinogenesis.Antioxid Redox Signal. 2001 Aug;3(4):597-609. doi: 10.1089/15230860152542952. Antioxid Redox Signal. 2001. PMID: 11554447 Review.
Cited by
-
Base excision repair and cancer.Cancer Lett. 2012 Dec 31;327(1-2):73-89. doi: 10.1016/j.canlet.2011.12.038. Epub 2012 Jan 15. Cancer Lett. 2012. PMID: 22252118 Free PMC article. Review.
-
Efficiency of Base Excision Repair of Oxidative DNA Damage and Its Impact on the Risk of Colorectal Cancer in the Polish Population.Oxid Med Cell Longev. 2016;2016:3125989. doi: 10.1155/2016/3125989. Epub 2015 Nov 16. Oxid Med Cell Longev. 2016. PMID: 26649135 Free PMC article.
-
Arsenic-induced genotoxicity and genetic susceptibility to arsenic-related pathologies.Int J Environ Res Public Health. 2013 Apr 12;10(4):1527-46. doi: 10.3390/ijerph10041527. Int J Environ Res Public Health. 2013. PMID: 23583964 Free PMC article. Review.
-
Gene-gene interactions in gastrointestinal cancer susceptibility.Onco_target. 2016 Oct 11;7(41):67612-67625. doi: 10.18632/onco_target.11701. Onco_target. 2016. PMID: 27588484 Free PMC article. Review.
-
OGG1 as an Epigenetic Reader Affects NFκB: What This Means for Cancer.Cancers (Basel). 2023 Dec 28;16(1):148. doi: 10.3390/cancers16010148. Cancers (Basel). 2023. PMID: 38201575 Free PMC article. Review.
References
-
- Doetsch P.W., Zasatawny T.H., Martin A.M., Dizdaroglu M. Monomeric base damage products from adenine, guanine, and thymine induced by exposure of DNA to ultraviolet radiation. Biochemistry. 1995;34:737–742. - PubMed
-
- Dizdaroglu M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry. 1985;24:4476–4481. - PubMed
-
- Kasai H., Nishimura S. Formation of 8-hydroxyguanosine in DNA by oxygen radicals and its biological significance. In: Seis H., editor. Oxidative Stress: Oxidants and Antioxidants. London: Academic Press; 1991. pp. 99–116.
-
- Cadet J., Berger M., Douki T., Ravanat J.L. Oxidative damage to DNA: formation, measurement, and biological significance. Rev. Physiol. Biochem. Pharmacol. 1997;131:1–87. - PubMed
-
- Barnes D.E., Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu. Rev. Genet. 2004;38:445–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

