Farnesoid X receptor is essential for normal glucose homeostasis
- PMID: 16557297
- PMCID: PMC1409738
- DOI: 10.1172/JCI25604
Farnesoid X receptor is essential for normal glucose homeostasis
Abstract
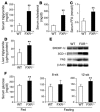
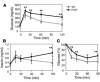
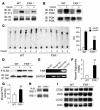
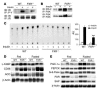
The bile acid receptor farnesoid X receptor (FXR; NR1H4) is a central regulator of bile acid and lipid metabolism. We show here that FXR plays a key regulatory role in glucose homeostasis. FXR-null mice developed severe fatty liver and elevated circulating FFAs, which was associated with elevated serum glucose and impaired glucose and insulin tolerance. Their insulin resistance was confirmed by the hyperinsulinemic euglycemic clamp, which showed attenuated inhibition of hepatic glucose production by insulin and reduced peripheral glucose disposal. In FXR-/- skeletal muscle and liver, multiple steps in the insulin signaling pathway were markedly blunted. In skeletal muscle, which does not express FXR, triglyceride and FFA levels were increased, and we propose that their inhibitory effects account for insulin resistance in that tissue. In contrast to the results in FXR-/- mice, bile acid activation of FXR in WT mice repressed expression of gluconeogenic genes and decreased serum glucose. The absence of this repression in both FXR-/- and small heterodimer partner-null (SHP-/-) mice demonstrated that the previously described FXR-SHP nuclear receptor cascade also _targets glucose metabolism. Taken together, our results identify a link between lipid and glucose metabolism mediated by the FXR-SHP cascade.
Figures


Similar articles
-
The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice.J Biol Chem. 2006 Apr 21;281(16):11039-49. doi: 10.1074/jbc.M510258200. Epub 2006 Jan 30. J Biol Chem. 2006. PMID: 16446356
-
Roux-en-Y Gastric Bypass Improves Metabolic Conditions in Association with Increased Serum Bile Acids Level and Hepatic Farnesoid X Receptor Expression in a T2DM Rat Model.Obes Surg. 2019 Sep;29(9):2912-2922. doi: 10.1007/s11695-019-03918-0. Obes Surg. 2019. PMID: 31079286
-
Hepatic FXR/SHP axis modulates systemic glucose and fatty acid homeostasis in aged mice.Hepatology. 2017 Aug;66(2):498-509. doi: 10.1002/hep.29199. Epub 2017 Jun 26. Hepatology. 2017. PMID: 28378930 Free PMC article.
-
FXR: a metabolic regulator and cell protector.Cell Res. 2008 Nov;18(11):1087-95. doi: 10.1038/cr.2008.289. Cell Res. 2008. PMID: 18825165 Review.
-
The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism.Arterioscler Thromb Vasc Biol. 2005 Oct;25(10):2020-30. doi: 10.1161/01.ATV.0000178994.21828.a7. Epub 2005 Jul 21. Arterioscler Thromb Vasc Biol. 2005. PMID: 16037564 Review.
Cited by
-
Intrahepatic Cholestasis of Pregnancy and Associated Adverse Maternal and Fetal Outcomes: A Retrospective Case-Control Study.Gastroenterol Res Pract. 2021 Mar 25;2021:6641023. doi: 10.1155/2021/6641023. eCollection 2021. Gastroenterol Res Pract. 2021. PMID: 33833795 Free PMC article.
-
The extended TILAR approach: a novel tool for dynamic modeling of the transcription factor network regulating the adaption to in vitro cultivation of murine hepatocytes.BMC Syst Biol. 2012 Nov 29;6:147. doi: 10.1186/1752-0509-6-147. BMC Syst Biol. 2012. PMID: 23190768 Free PMC article.
-
A meta-analysis of the prevalence of gestational diabetes in patients diagnosed with obstetrical cholestasis.AJOG Glob Rep. 2021 Jun 14;1(3):100013. doi: 10.1016/j.xagr.2021.100013. eCollection 2021 Aug. AJOG Glob Rep. 2021. PMID: 36277255 Free PMC article.
-
Cholesterol 7α-hydroxylase-deficient mice are protected from high-fat/high-cholesterol diet-induced metabolic disorders.J Lipid Res. 2016 Jul;57(7):1144-54. doi: 10.1194/jlr.M064709. Epub 2016 May 4. J Lipid Res. 2016. PMID: 27146480 Free PMC article.
-
The Liver under the Spotlight: Bile Acids and Oxysterols as Pivotal Actors Controlling Metabolism.Cells. 2021 Feb 16;10(2):400. doi: 10.3390/cells10020400. Cells. 2021. PMID: 33669184 Free PMC article. Review.
References
-
- Parks D.J., et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. - PubMed
-
- Makishima M., et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. - PubMed
-
- Wang H., Chen J., Hollister K., Sowers L.C., Forman B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 1999;3:543–553. - PubMed
-
- Goodwin B., et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 2000;6:517–526. - PubMed
-
- Wang L., et al. Redundant pathways for negative feedback regulation of bile acid production. Dev. Cell. 2002;2:721–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

