Crystal structure of the CorA Mg2+ transporter
- PMID: 16598263
- PMCID: PMC3836678
- DOI: 10.1038/nature04642
Crystal structure of the CorA Mg2+ transporter
Abstract
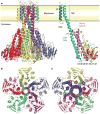
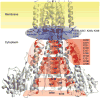
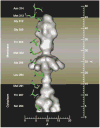
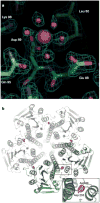
The magnesium ion, Mg2+, is essential for myriad biochemical processes and remains the only major biological ion whose transport mechanisms remain unknown. The CorA family of magnesium transporters is the primary Mg2+ uptake system of most prokaryotes and a functional homologue of the eukaryotic mitochondrial magnesium transporter. Here we determine crystal structures of the full-length Thermotoga maritima CorA in an apparent closed state and its isolated cytoplasmic domain at 3.9 A and 1.85 A resolution, respectively. The transporter is a funnel-shaped homopentamer with two transmembrane helices per monomer. The channel is formed by an inner group of five helices and putatively gated by bulky hydrophobic residues. The large cytoplasmic domain forms a funnel whose wide mouth points into the cell and whose walls are formed by five long helices that are extensions of the transmembrane helices. The cytoplasmic neck of the pore is surrounded, on the outside of the funnel, by a ring of highly conserved positively charged residues. Two negatively charged helices in the cytoplasmic domain extend back towards the membrane on the outside of the funnel and abut the ring of positive charge. An apparent Mg2+ ion was bound between monomers at a conserved site in the cytoplasmic domain, suggesting a mechanism to link gating of the pore to the intracellular concentration of Mg2+.
Figures
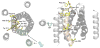
Similar articles
-
Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution.Science. 2006 Jul 21;313(5785):354-7. doi: 10.1126/science.1127121. Science. 2006. PMID: 16857941
-
The structure of CorA: a Mg(2+)-selective channel.Curr Opin Struct Biol. 2006 Aug;16(4):432-8. doi: 10.1016/j.sbi.2006.06.006. Epub 2006 Jul 7. Curr Opin Struct Biol. 2006. PMID: 16828282 Review.
-
Exploring the structure and function of Thermotoga maritima CorA reveals the mechanism of gating and ion selectivity in Co2+/Mg2+ transport.Biochem J. 2013 May 1;451(3):365-74. doi: 10.1042/BJ20121745. Biochem J. 2013. PMID: 23425532 Free PMC article.
-
Crystal structure of the MgtE Mg2+ transporter.Nature. 2007 Aug 30;448(7157):1072-5. doi: 10.1038/nature06093. Epub 2007 Aug 15. Nature. 2007. PMID: 17700703
-
Magnesium transporters: properties, regulation and structure.Front Biosci. 2006 Sep 1;11:3149-63. doi: 10.2741/2039. Front Biosci. 2006. PMID: 16720382 Review.
Cited by
-
Initial binding of ions to the interhelical loops of divalent ion transporter CorA: replica exchange molecular dynamics simulation study.PLoS One. 2012;7(8):e43872. doi: 10.1371/journal.pone.0043872. Epub 2012 Aug 30. PLoS One. 2012. PMID: 22952795 Free PMC article.
-
A structural basis for Mg2+ homeostasis and the CorA translocation cycle.EMBO J. 2006 Aug 23;25(16):3762-73. doi: 10.1038/sj.emboj.7601269. Epub 2006 Aug 10. EMBO J. 2006. PMID: 16902408 Free PMC article.
-
Loss of cytosolic Mg2+ binding sites in the Thermotoga maritima CorA Mg2+ channel is not sufficient for channel opening.Biochim Biophys Acta Gen Subj. 2019 Jan;1863(1):25-30. doi: 10.1016/j.bbagen.2018.09.001. Epub 2018 Sep 5. Biochim Biophys Acta Gen Subj. 2019. PMID: 30293964 Free PMC article.
-
Physiological Essence of Magnesium in Plants and Its Widespread Deficiency in the Farming System of China.Front Plant Sci. 2022 Apr 25;13:802274. doi: 10.3389/fpls.2022.802274. eCollection 2022. Front Plant Sci. 2022. PMID: 35548291 Free PMC article. Review.
-
Structural dynamics of the magnesium-bound conformation of CorA in a lipid bilayer.Structure. 2010 Jul 14;18(7):868-78. doi: 10.1016/j.str.2010.04.009. Structure. 2010. PMID: 20637423 Free PMC article.
References
-
- Nelson DL, Kennedy EP. Magnesium transport in Escherichia coli Inhibition by cobaltous ion. J Biol Chem. 1971;246:3042–3049. - PubMed
-
- Bui DM, Gregan J, Jarosch E, Ragnini A, Schweyen RJ. The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondrial membrane. J Biol Chem. 1999;274:20438–20443. - PubMed
-
- Kehres DG, Maguire ME. Structure, properties and regulation of magnesium transport proteins. Biometals. 2002;15:261–270. - PubMed
-
- Kehres DG, Lawyer CH, Maguire ME. The CorA magnesium transporter gene family. Microb Compar Genom. 1998;43:151–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

