BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype
- PMID: 16598384
- PMCID: PMC2435171
- DOI: 10.1359/jbmr.060109
BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype
Abstract
RUNX2 expression in mesenchymal cells induces osteoblast differentiation and bone formation. BMP blocking agents were used to show that RUNX2-dependent osteoblast differentiation and transactivation activity both require BMP signaling and, further, that RUNX2 enhances the responsiveness of cells to BMPs.
Introduction: BMPs and the RUNX2 transcription factor are both able to stimulate osteoblast differentiation and bone formation. BMPs function by activating SMAD proteins and other signal transduction pathways to stimulate expression of many _target genes including RUNX2. In contrast, RUNX2 induces osteoblast-specific gene expression by directly binding to enhancer regions in _target genes. In this study, we examine the interdependence of these two factors in controlling osteoblast differentiation in mesenchymal progenitor cells.
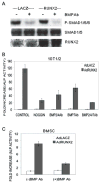
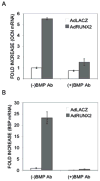
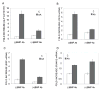
Materials and methods: C3H10T1/2 mesenchymal cells and primary cultures of marrow stromal cells were transduced with a RUNX2 adenovirus and treated with BMP blocking antibodies or the natural antagonist, NOGGIN. Osteoblast differentiation was determined by assaying alkaline phosphatase and measuring osteoblast-related mRNA using quantitative RT/PCR. Activation of BMP-responsive signal transduction pathways (SMAD, extracellular signal-regulated kinase [ERK], p38, and c-jun-N-terminal kinase [JNK]) was assessed on Western blots.
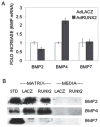
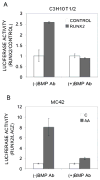
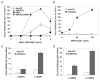
Results and conclusions: C3H10T1/2 cells constitutively synthesize BMP2 and 4 mRNA and protein, and this BMP activity is sufficient to activate basal levels of SMAD phosphorylation. Inhibition of BMP signaling was shown to disrupt the ability of RUNX2 to stimulate osteoblast differentiation and transactivate an osteocalcin gene promoter-luciferase reporter in C3H10T1/2 cells. BMP blocking antibodies also inhibited RUNX2-dependent osteoblast differentiation in primary cultures of murine marrow stromal cells. Conversely, RUNX2 expression synergistically stimulated BMP2 signaling in C3H10T1/2 cells. However, RUNX2 did not increase the ability of this BMP to activate SMAD, ERK, p38, and JNK pathways. This study shows that autocrine BMP production is necessary for the RUNX2 transcription factor to be active and that BMPs and RUNX2 cooperatively interact to stimulate osteoblast gene expression.
Conflict of interest statement
The authors state that they have no conflicts of interest.
Figures

Similar articles
-
Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts.J Bone Miner Res. 2007 Jun;22(6):918-30. doi: 10.1359/jbmr.070312. J Bone Miner Res. 2007. PMID: 17352654 Free PMC article.
-
Reconstitution of Runx2/Cbfa1-null cells identifies a requirement for BMP2 signaling through a Runx2 functional domain during osteoblast differentiation.J Cell Biochem. 2007 Feb 1;100(2):434-49. doi: 10.1002/jcb.21039. J Cell Biochem. 2007. PMID: 16927309
-
Harmine promotes osteoblast differentiation through bone morphogenetic protein signaling.Biochem Biophys Res Commun. 2011 Jun 3;409(2):260-5. doi: 10.1016/j.bbrc.2011.05.001. Epub 2011 May 6. Biochem Biophys Res Commun. 2011. PMID: 21570953
-
Smad-Runx interactions during chondrocyte maturation.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S15-22. J Bone Joint Surg Am. 2001. PMID: 11263661 Review.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
Cited by
-
Physicochemical properties and cell proliferation and adhesive bioactivity of collagen-hyaluronate composite gradient membrane.Front Bioeng Biotechnol. 2023 Oct 25;11:1287359. doi: 10.3389/fbioe.2023.1287359. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37954023 Free PMC article.
-
Transcriptomic Analysis of Mineralized Adipose-Derived Stem Cell Tissues for Calcific Valve Disease Modelling.Int J Mol Sci. 2024 Feb 14;25(4):2291. doi: 10.3390/ijms25042291. Int J Mol Sci. 2024. PMID: 38396969 Free PMC article.
-
Effects of Scytosiphon lomentaria on osteoblastic proliferation and differentiation of MC3T3-E1 cells.Nutr Res Pract. 2016 Apr;10(2):148-53. doi: 10.4162/nrp.2016.10.2.148. Epub 2016 Mar 2. Nutr Res Pract. 2016. PMID: 27087897 Free PMC article.
-
USP36 regulates the proliferation, survival, and differentiation of hFOB1.19 osteoblast.J Orthop Surg Res. 2024 Aug 17;19(1):483. doi: 10.1186/s13018-024-04893-8. J Orthop Surg Res. 2024. PMID: 39152465 Free PMC article.
-
Trifloroside Induces Bioactive Effects on Differentiation, Adhesion, Migration, and Mineralization in Pre-Osteoblast MC3T3E-1 Cells.Cells. 2022 Dec 1;11(23):3887. doi: 10.3390/cells11233887. Cells. 2022. PMID: 36497145 Free PMC article.
References
-
- Katagiri T, Yamaguchi A, Ikeda T, Yoshiki S, Wozney JM, Rosen V, Wang EA, Tanaka H, Omura S, Suda T. The non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochem Biophys Res Commun. 1990;172:295–299. - PubMed
-
- Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992;74:659–670. - PubMed
-
- Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

