Adenosine A2a blockade prevents synergy between mu-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats
- PMID: 16684876
- PMCID: PMC1458620
- DOI: 10.1073/pnas.0602661103
Adenosine A2a blockade prevents synergy between mu-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats
Abstract
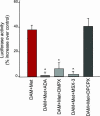
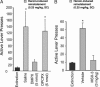
Relapse is the most serious limitation of effective medical treatment of opiate addiction. Opiate-related behaviors appear to be modulated by cannabinoid CB1 receptors (CB1) through poorly understood cross-talk mechanisms. Opiate and CB1 receptors are coexpressed in the nucleus accumbens (NAc) and dorsal striatum. These regions also have the highest density of adenosine A2a receptors (A2a) in the brain. We have been investigating the postsynaptic signaling mechanisms of mu-opiate receptors (MORs) and CB1 receptors in primary NAc/striatal neurons. In this article, we present evidence that MOR and CB1 act synergistically on cAMP/PKA signaling in NAc/striatal neurons. In addition, we find that synergy requires adenosine and A2a. Importantly, an A2a antagonist administered either directly into the NAc or indirectly by i.p. injection eliminates heroin-induced reinstatement in rats trained to self-administer heroin, a model of human craving and relapse. These findings suggest that A2a antagonists might be effective therapeutic agents in the management of abstinent heroin addicts.
Conflict of interest statement
Conflict of interest statement: A patent application concerning this work is under review and will be owned by the University of California at San Francisco. I.D. is Vice President and L.Y. is a Senior Scientist at CV Therapeutics, Inc.
Figures

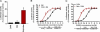
Similar articles
-
Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2.Eur J Neurosci. 2007 Apr;25(7):2191-200. doi: 10.1111/j.1460-9568.2007.05470.x. Eur J Neurosci. 2007. PMID: 17419755
-
Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist.Addict Biol. 2011 Jul;16(3):405-15. doi: 10.1111/j.1369-1600.2010.00258.x. Epub 2010 Nov 4. Addict Biol. 2011. PMID: 21054689 Free PMC article.
-
Mu-opioid and CB1 cannabinoid receptors of the dorsal periaqueductal gray interplay in the regulation of fear response, but not antinociception.Pharmacol Biochem Behav. 2020 Jul;194:172938. doi: 10.1016/j.pbb.2020.172938. Epub 2020 May 3. Pharmacol Biochem Behav. 2020. PMID: 32376258
-
Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain.Prog Neurobiol. 2007 Dec;83(5):332-47. doi: 10.1016/j.pneurobio.2007.04.002. Epub 2007 May 1. Prog Neurobiol. 2007. PMID: 17532111 Free PMC article. Review.
-
Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal.Neuroscience. 2013 Sep 17;248:637-54. doi: 10.1016/j.neuroscience.2013.04.034. Epub 2013 Apr 24. Neuroscience. 2013. PMID: 23624062 Free PMC article. Review.
Cited by
-
Behavioural and biochemical responses to morphine associated with its motivational properties are altered in adenosine A(2A) receptor knockout mice.Br J Pharmacol. 2008 Nov;155(5):757-66. doi: 10.1038/bjp.2008.299. Epub 2008 Jul 28. Br J Pharmacol. 2008. PMID: 18660831 Free PMC article.
-
Cannabinoids in health and disease.Dialogues Clin Neurosci. 2007;9(4):413-30. doi: 10.31887/DCNS.2007.9.4/nkogan. Dialogues Clin Neurosci. 2007. PMID: 18286801 Free PMC article. Review.
-
Presynaptic adenosine A2A receptors dampen cannabinoid CB1 receptor-mediated inhibition of corticostriatal glutamatergic transmission.Br J Pharmacol. 2015 Feb;172(4):1074-86. doi: 10.1111/bph.12970. Epub 2015 Jan 12. Br J Pharmacol. 2015. PMID: 25296982 Free PMC article.
-
Activating A1 adenosine receptor signaling boosts early pulmonary neutrophil recruitment in aged mice in response to Streptococcus pneumoniae infection.Immun Ageing. 2024 Jun 5;21(1):34. doi: 10.1186/s12979-024-00442-3. Immun Ageing. 2024. PMID: 38840213 Free PMC article.
-
Heteromerization of G protein-coupled receptors: relevance to neurological disorders and neurotherapeutics.CNS Neurol Disord Drug _targets. 2010 Nov;9(5):636-50. doi: 10.2174/187152710793361586. CNS Neurol Disord Drug _targets. 2010. PMID: 20632964 Free PMC article. Review.
References
-
- Pan Z. Z. Trends Pharmacol. Sci. 1998;19:94–98. - PubMed
-
- Negus S. S., Burke T. F., Medzihradsky F., Woods J. H. J. Pharmacol. Exp. Ther. 1993;267:896–903. - PubMed
-
- Matthes H. W., Maldonado R., Simonin F., Valverde O., Slowe S., Kitchen I., Befort K., Dierich A., Le Meur M., Dolle P., et al. Nature. 1996;383:819–823. - PubMed
-
- Alderson H. L., Parkinson J. A., Robbins T. W., Everitt B. J. Psychopharmacology. 2001;153:455–463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

