HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane
- PMID: 16699598
- PMCID: PMC1458960
- DOI: 10.1371/journal.ppat.0020039
HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane
Abstract
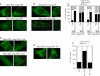
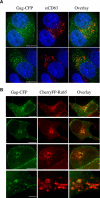
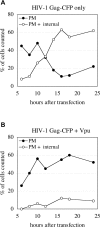
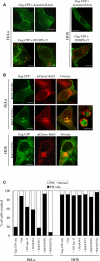
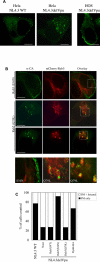
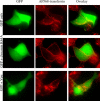
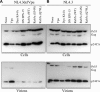
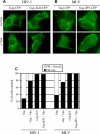
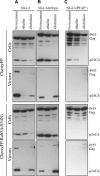
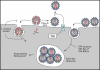
The human immunodeficiency virus (HIV) type-1 viral protein U (Vpu) protein enhances the release of diverse retroviruses from human, but not monkey, cells and is thought to do so by ablating a dominant restriction to particle release. Here, we determined how Vpu expression affects the subcellular distribution of HIV-1 and murine leukemia virus (MLV) Gag proteins in human cells where Vpu is, or is not, required for efficient particle release. In HeLa cells, where Vpu enhances HIV-1 and MLV release approximately 10-fold, concentrations of HIV-1 Gag and MLV Gag fused to cyan fluorescent protein (CFP) were initially detected at the plasma membrane, but then accumulated over time in early and late endosomes. Endosomal accumulation of Gag-CFP was prevented by Vpu expression and, importantly, inhibition of plasma membrane to early endosome transport by dominant negative mutants of Rab5a, dynamin, and EPS-15. Additionally, accumulation of both HIV and MLV Gag in endosomes required a functional late-budding domain. In human HOS cells, where HIV-1 and MLV release was efficient even in the absence of Vpu, Gag proteins were localized predominantly at the plasma membrane, irrespective of Vpu expression or manipulation of endocytic transport. While these data indicated that Vpu inhibits nascent virion endocytosis, Vpu did not affect transferrin endocytosis. Moreover, inhibition of endocytosis did not restore Vpu-defective HIV-1 release in HeLa cells, but instead resulted in accumulation of mature virions that could be released from the cell surface by protease treatment. Thus, these findings suggest that a specific activity that is present in HeLa cells, but not in HOS cells, and is counteracted by Vpu, traps assembled retrovirus particles at the cell surface. This entrapment leads to subsequent endocytosis by a Rab5a- and clathrin-dependent mechanism and intracellular sequestration of virions in endosomes.
Conflict of interest statement
Figures


Similar articles
-
HIV-1 Vpu inhibits accumulation of the envelope glycoprotein within clathrin-coated, Gag-containing endosomes.Cell Microbiol. 2008 May;10(5):1040-57. doi: 10.1111/j.1462-5822.2007.01101.x. Epub 2007 Dec 10. Cell Microbiol. 2008. PMID: 18076669
-
Vpu and Tsg101 regulate intracellular _targeting of the human immunodeficiency virus type 1 core protein precursor Pr55gag.J Virol. 2006 Apr;80(8):3765-72. doi: 10.1128/JVI.80.8.3765-3772.2006. J Virol. 2006. PMID: 16571793 Free PMC article.
-
The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein.Cell Host Microbe. 2008 Apr 17;3(4):245-52. doi: 10.1016/j.chom.2008.03.001. Epub 2008 Mar 13. Cell Host Microbe. 2008. PMID: 18342597 Free PMC article.
-
Tetherin/BST-2: Restriction Factor or Immunomodulator?Curr HIV Res. 2016;14(3):235-46. doi: 10.2174/1570162x14999160224102752. Curr HIV Res. 2016. PMID: 26957198 Free PMC article. Review.
-
Assembling the human immunodeficiency virus type 1.Cell Mol Life Sci. 2002 Jul;59(7):1166-84. doi: 10.1007/s00018-002-8495-6. Cell Mol Life Sci. 2002. PMID: 12222963 Free PMC article. Review.
Cited by
-
Imaging HIV-1 RNA dimerization in cells by multicolor super-resolution and fluctuation microscopies.Nucleic Acids Res. 2016 Sep 19;44(16):7922-34. doi: 10.1093/nar/gkw511. Epub 2016 Jun 8. Nucleic Acids Res. 2016. PMID: 27280976 Free PMC article.
-
Major histocompatibility complex class-II molecules promote _targeting of human immunodeficiency virus type 1 virions in late endosomes by enhancing internalization of nascent particles from the plasma membrane.Cell Microbiol. 2013 May;15(5):809-22. doi: 10.1111/cmi.12074. Epub 2012 Dec 12. Cell Microbiol. 2013. PMID: 23170932 Free PMC article.
-
Tetherin restricts productive HIV-1 cell-to-cell transmission.PLoS Pathog. 2010 Jun 17;6(6):e1000955. doi: 10.1371/journal.ppat.1000955. PLoS Pathog. 2010. PMID: 20585562 Free PMC article.
-
Directional spread of surface-associated retroviruses regulated by differential virus-cell interactions.J Virol. 2010 Apr;84(7):3248-58. doi: 10.1128/JVI.02155-09. Epub 2010 Jan 20. J Virol. 2010. PMID: 20089647 Free PMC article.
-
Separable determinants of subcellular localization and interaction account for the inability of group O HIV-1 Vpu to counteract tetherin.J Virol. 2011 Oct;85(19):9737-48. doi: 10.1128/JVI.00479-11. Epub 2011 Jul 20. J Virol. 2011. PMID: 21775465 Free PMC article.
References
-
- Cohen EA, Terwilliger EF, Sodroski JG, Haseltine WA. Identification of a protein encoded by the Vpu gene of HIV-1. Nature. 1988;334:532–534. - PubMed
-
- Strebel K, Klimkait T, Martin MA. A novel gene of HIV-1, Vpu, and its 16-kilodalton product. Science. 1988;241:1221–1223. - PubMed
-
- Huet T, Cheynier R, Meyerhans A, Roelants G, Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990;345:356–359. - PubMed
-
- Courgnaud V, Salemi M, Pourrut X, Mpoudi-Ngole E, Abela B, et al. Characterization of a novel simian immunodeficiency virus with a Vpu gene from greater spot-nosed monkeys (Cercopithecus nictitans) provides new insights into simian/human immunodeficiency virus phylogeny. J Virol. 2002;76:8298–8309. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

