Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors
- PMID: 16710478
- PMCID: PMC1462942
- DOI: 10.1172/JCI27114
Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors
Abstract
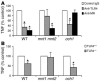
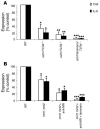
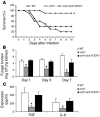
The fungal pathogen Candida albicans has a multilayered cell wall composed of an outer layer of proteins glycosylated with N- or O-linked mannosyl residues and an inner skeletal layer of beta-glucans and chitin. We demonstrate that cytokine production by human mononuclear cells or murine macrophages was markedly reduced when stimulated by C. albicans mutants defective in mannosylation. Recognition of mannosyl residues was mediated by mannose receptor binding to N-linked mannosyl residues and by TLR4 binding to O-linked mannosyl residues. Residual cytokine production was mediated by recognition of beta-glucan by the dectin-1/TLR2 receptor complex. C. albicans mutants with a cell wall defective in mannosyl residues were less virulent in experimental disseminated candidiasis and elicited reduced cytokine production in vivo. We concluded that recognition of C. albicans by monocytes/macrophages is mediated by 3 recognition systems of differing importance, each of which senses specific layers of the C. albicans cell wall.
Figures

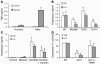
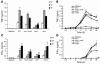
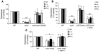
Similar articles
-
Immune recognition of Candida albicans beta-glucan by dectin-1.J Infect Dis. 2007 Nov 15;196(10):1565-71. doi: 10.1086/523110. Epub 2007 Oct 31. J Infect Dis. 2007. PMID: 18008237 Free PMC article.
-
Candida albicans N-Linked Mannans Potentiate the Induction of Trained Immunity via Dectin-2.J Infect Dis. 2024 Sep 23;230(3):768-777. doi: 10.1093/infdis/jiae112. J Infect Dis. 2024. PMID: 38446996
-
Mnn10 Maintains Pathogenicity in Candida albicans by Extending α-1,6-Mannose Backbone to Evade Host Dectin-1 Mediated Antifungal Immunity.PLoS Pathog. 2016 May 4;12(5):e1005617. doi: 10.1371/journal.ppat.1005617. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27144456 Free PMC article.
-
Mannosylation in Candida albicans: role in cell wall function and immune recognition.Mol Microbiol. 2013 Dec;90(6):1147-61. doi: 10.1111/mmi.12426. Epub 2013 Nov 8. Mol Microbiol. 2013. PMID: 24125554 Free PMC article. Review.
-
The Candida albicans phosphomannan complex in Candida-host interactions.Res Immunol. 1998 May-Jun;149(4-5):299-308; discussion 507-9. doi: 10.1016/s0923-2494(98)80754-2. Res Immunol. 1998. PMID: 9720948 Review. No abstract available.
Cited by
-
Fungal pathogens-a sweet and sour treat for toll-like receptors.Front Cell Infect Microbiol. 2012 Nov 22;2:142. doi: 10.3389/fcimb.2012.00142. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23189270 Free PMC article. Review.
-
Microsporum gypseum Isolated from Ailuropoda melanoleuca Provokes Inflammation and Triggers Th17 Adaptive Immunity Response.Int J Mol Sci. 2022 Oct 10;23(19):12037. doi: 10.3390/ijms231912037. Int J Mol Sci. 2022. PMID: 36233337 Free PMC article.
-
The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections.Eur J Clin Microbiol Infect Dis. 2015 May;34(5):963-974. doi: 10.1007/s10096-014-2309-2. Epub 2015 Jan 13. Eur J Clin Microbiol Infect Dis. 2015. PMID: 25579795 Free PMC article.
-
Quantitative and qualitative analysis of the antifungal activity of allicin alone and in combination with antifungal drugs.PLoS One. 2012;7(6):e38242. doi: 10.1371/journal.pone.0038242. Epub 2012 Jun 5. PLoS One. 2012. PMID: 22679493 Free PMC article.
-
The role of galectin-3 in phagocytosis of Candida albicans and Candida parapsilosis by human neutrophils.Cell Microbiol. 2013 Jul;15(7):1127-42. doi: 10.1111/cmi.12103. Epub 2013 Jan 20. Cell Microbiol. 2013. PMID: 23279221 Free PMC article.
References
-
- Pfaller M.A., et al. International surveillance of blood stream infections due to Candida species in the European SENTRY Program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 1999;35:19–25. - PubMed
-
- Edmond M.B., et al. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 1999;29:239–244. - PubMed
-
- Van ‘t Wout J.W., Linde I., Leijh P.C.J., Van Furth R. Contribution of granulocytes and monocytes to resistance against experimental disseminatedCandida albicans infections. . Eur. J. Clin. Microbiol. Infect. Dis. 1988;7:736–741. - PubMed
-
- Marodi L., Korchak H.M., Johnston R.B. Mechanisms of host defense againstCandida species. 1. Phagocytosis by monocytes and monocyte-derived macrophages. . J. Immunol. 1991;146:2783–2789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

