Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta
- PMID: 16880506
- PMCID: PMC1592798
- DOI: 10.1128/MCB.00383-06
Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta
Abstract
AMP-activated protein kinase (AMPK) is a sensor of cellular energy state in response to metabolic stress and other regulatory signals. AMPK is controlled by upstream kinases which have recently been identified as LKB1 or Ca2+/calmodulin-dependent protein kinase kinase beta (CaMKKbeta). Our study of human endothelial cells shows that AMPK is activated by thrombin through a Ca2+-dependent mechanism involving the thrombin receptor protease-activated receptor 1 and Gq-protein-mediated phospholipase C activation. Inhibition of CaMKK with STO-609 or downregulation of CaMKKbeta using RNA interference decreased thrombin-induced AMPK activation significantly, indicating that CaMKKbeta was the responsible AMPK kinase. In contrast, downregulation of LKB1 did not affect thrombin-induced AMPK activation but abolished phosphorylation of AMPK with 5-aminoimidazole-4-carboxamide ribonucleoside. Thrombin stimulation led to phosphorylation of acetyl coenzyme A carboxylase (ACC) and endothelial nitric oxide synthase (eNOS), two downstream _targets of AMPK. Inhibition or downregulation of CaMKKbeta or AMPK abolished phosphorylation of ACC in response to thrombin but had no effect on eNOS phosphorylation, indicating that thrombin-stimulated phosphorylation of eNOS is not mediated by AMPK. Our results underline the role of Ca2+ as a regulator of AMPK activation in response to a physiologic stimulation. We also demonstrate that endothelial cells possess two pathways to activate AMPK, one Ca2+/CaMKKbeta dependent and one AMP/LKB1 dependent.
Figures
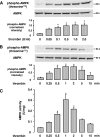
 , P < 0.05 versus untreated controls.
, P < 0.05 versus untreated controls.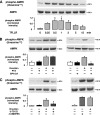
 , P < 0.05.
, P < 0.05.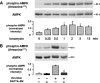
 , P < 0.05.
, P < 0.05.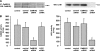
 , P < 0.05 versus control siRNA.
, P < 0.05 versus control siRNA.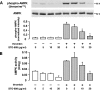
 , P < 0.05 versus untreated thrombin-stimulated controls.
, P < 0.05 versus untreated thrombin-stimulated controls.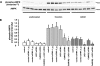
 , P < 0.05 versus samples pretreated with control siRNA and stimulated with thrombin or AICAR.
, P < 0.05 versus samples pretreated with control siRNA and stimulated with thrombin or AICAR.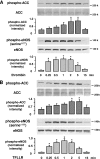
 , P < 0.05 versus untreated controls.
, P < 0.05 versus untreated controls.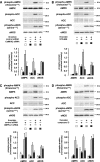
 , P < 0.05 versus thrombin-stimulated samples pretreated with control siRNA (A and C) or solvent (B and D).
, P < 0.05 versus thrombin-stimulated samples pretreated with control siRNA (A and C) or solvent (B and D).Similar articles
-
eNOS activation mediated by AMPK after stimulation of endothelial cells with histamine or thrombin is dependent on LKB1.Biochim Biophys Acta. 2011 Feb;1813(2):322-31. doi: 10.1016/j.bbamcr.2010.12.001. Epub 2010 Dec 9. Biochim Biophys Acta. 2011. PMID: 21145922
-
Mechanism of thrombin mediated eNOS phosphorylation in endothelial cells is dependent on ATP levels after stimulation.Biochim Biophys Acta. 2008 Oct;1783(10):1893-902. doi: 10.1016/j.bbamcr.2008.07.003. Epub 2008 Jul 18. Biochim Biophys Acta. 2008. PMID: 18687367
-
Estrogen Activates AMP-Activated Protein Kinase in Human Endothelial Cells via ERβ/Ca(2+)/Calmodulin-Dependent Protein Kinase Kinase β Pathway.Cell Biochem Biophys. 2015 Jul;72(3):701-7. doi: 10.1007/s12013-015-0521-z. Cell Biochem Biophys. 2015. PMID: 25616441
-
AMP-activated protein kinase in metabolic control and insulin signaling.Circ Res. 2007 Feb 16;100(3):328-41. doi: 10.1161/01.RES.0000256090.42690.05. Circ Res. 2007. PMID: 17307971 Review.
-
AMP-activated protein kinase: implications on ischemic diseases.BMB Rep. 2012 Sep;45(9):489-95. doi: 10.5483/bmbrep.2012.45.9.169. BMB Rep. 2012. PMID: 23010169 Review.
Cited by
-
Activation of the AMP-activated protein kinase (AMPK) by nitrated lipids in endothelial cells.PLoS One. 2012;7(2):e31056. doi: 10.1371/journal.pone.0031056. Epub 2012 Feb 17. PLoS One. 2012. Retraction in: PLoS One. 2020 May 19;15(5):e0233408. doi: 10.1371/journal.pone.0233408 PMID: 22363546 Free PMC article. Retracted.
-
Autophagy as a Guardian of Vascular Niche Homeostasis.Int J Mol Sci. 2024 Sep 20;25(18):10097. doi: 10.3390/ijms251810097. Int J Mol Sci. 2024. PMID: 39337582 Free PMC article. Review.
-
NorUDCA promotes degradation of α1-antitrypsin mutant Z protein by inducing autophagy through AMPK/ULK1 pathway.PLoS One. 2018 Aug 1;13(8):e0200897. doi: 10.1371/journal.pone.0200897. eCollection 2018. PLoS One. 2018. PMID: 30067827 Free PMC article.
-
The Roles of AMPK in Revascularization.Cardiol Res Pract. 2020 Feb 27;2020:4028635. doi: 10.1155/2020/4028635. eCollection 2020. Cardiol Res Pract. 2020. PMID: 32185076 Free PMC article. Review.
-
Amino Acids in Cell Signaling: Regulation and Function.Adv Exp Med Biol. 2021;1332:17-33. doi: 10.1007/978-3-030-74180-8_2. Adv Exp Med Biol. 2021. PMID: 34251636
References
-
- Aley, P. K., K. E. Porter, J. P. Boyle, P. J. Kemp, and C. Peers. 2005. Hypoxic modulation of Ca2+ signaling in human venous endothelial cells. Multiple roles for reactive oxygen species. J. Biol. Chem. 280:13349-13354. - PubMed
-
- Reference deleted.
-
- Bogatcheva, N. V., J. G. Garcia, and A. D. Verin. 2002. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry (Moscow) 67:75-84. - PubMed
-
- Borbiev, T., A. D. Verin, S. Shi, F. Liu, and J. G. Garcia. 2001. Regulation of endothelial cell barrier function by calcium/calmodulin-dependent protein kinase II. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L983-L990. - PubMed
-
- Cacicedo, J. M., N. Yagihashi, J. F. Keaney, Jr., N. B. Ruderman, and Y. Ido. 2004. AMPK inhibits fatty acid-induced increases in NF-κB transactivation in cultured human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 324:1204-1209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
