Candida albicans biofilms produce antifungal-tolerant persister cells
- PMID: 16923951
- PMCID: PMC1635216
- DOI: 10.1128/AAC.00684-06
Candida albicans biofilms produce antifungal-tolerant persister cells
Abstract
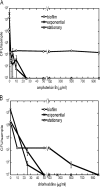
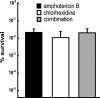
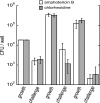
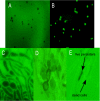
Fungal pathogens form biofilms that are highly recalcitrant to antimicrobial therapy. The expression of multidrug resistance pumps in young biofilms has been linked to increased resistance to azoles, but this mechanism does not seem to underlie the resistance of mature biofilms that is a model of in vivo infection. The mechanism of drug resistance of mature biofilms remains largely unknown. We report that biofilms formed by the major human pathogen Candida albicans exhibited a strikingly biphasic killing pattern in response to two microbicidal agents, amphotericin B, a polyene antifungal, and chlorhexidine, an antiseptic, indicating that a subpopulation of highly tolerant cells, termed persisters, existed. The extent of killing with a combination of amphotericin B and chlorhexidine was similar to that observed with individually added antimicrobials. Thus, surviving persisters form a multidrug-tolerant subpopulation. Interestingly, surviving C. albicans persisters were detected only in biofilms and not in exponentially growing or stationary-phase planktonic populations. Reinoculation of cells that survived killing of the biofilm by amphotericin B produced a new biofilm with a new subpopulation of persisters. This suggests that C. albicans persisters are not mutants but phenotypic variants of the wild type. Using a stain for dead cells, rare dark cells were visible in a biofilm after amphotericin B treatment, and a bright and a dim population were physically sorted from this biofilm. Only the dim cells produced colonies, showing that this method allows the isolation of yeast persisters. Given that persisters formed only in biofilms, mutants defective in biofilm formation were examined for tolerance of amphotericin B. All of the known mutants affected in biofilm formation were able to produce normal levels of persisters. This finding indicates that attachment rather than formation of a complex biofilm architecture initiates persister formation. Bacteria produce multidrug-tolerant persister cells in both planktonic and biofilm populations, and it appears that yeasts and bacteria have evolved analogous strategies that assign the function of survival to a small part of the population. In bacteria, persisters are dormant cells. It remains to be seen whether attachment initiates dormancy that leads to the formation of fungal persisters. This study suggests that persisters may be largely responsible for the multidrug tolerance of fungal biofilms.
Figures

Similar articles
-
Inhibition of nucleic acid biosynthesis makes little difference to formation of amphotericin B-tolerant persisters in Candida albicans biofilm.Antimicrob Agents Chemother. 2015 Mar;59(3):1627-33. doi: 10.1128/AAC.03765-14. Epub 2014 Dec 29. Antimicrob Agents Chemother. 2015. PMID: 25547355 Free PMC article.
-
Protocol for Determination of the Persister Subpopulation in Candida Albicans Biofilms.Methods Mol Biol. 2016;1333:67-72. doi: 10.1007/978-1-4939-2854-5_6. Methods Mol Biol. 2016. PMID: 26468100
-
Candida albicans Amphotericin B-Tolerant Persister Formation is Closely Related to Surface Adhesion.Mycopathologia. 2016 Feb;181(1-2):41-9. doi: 10.1007/s11046-015-9894-1. Epub 2015 Sep 18. Mycopathologia. 2016. PMID: 26381156
-
Multidrug tolerance of biofilms and persister cells.Curr Top Microbiol Immunol. 2008;322:107-31. doi: 10.1007/978-3-540-75418-3_6. Curr Top Microbiol Immunol. 2008. PMID: 18453274 Review.
-
Fungal persister cells: The basis for recalcitrant infections?PLoS Pathog. 2018 Oct 18;14(10):e1007301. doi: 10.1371/journal.ppat.1007301. eCollection 2018 Oct. PLoS Pathog. 2018. PMID: 30335865 Free PMC article. Review.
Cited by
-
Pharmacodynamics, population dynamics, and the evolution of persistence in Staphylococcus aureus.PLoS Genet. 2013;9(1):e1003123. doi: 10.1371/journal.pgen.1003123. Epub 2013 Jan 3. PLoS Genet. 2013. PMID: 23300474 Free PMC article.
-
Visible Lights Combined with Photosensitizing Compounds Are Effective against Candida albicans Biofilms.Microorganisms. 2021 Feb 26;9(3):500. doi: 10.3390/microorganisms9030500. Microorganisms. 2021. PMID: 33652865 Free PMC article.
-
Candida albicans Biofilm Development and Its Genetic Control.Microbiol Spectr. 2015 Jun;3(3):10.1128/microbiolspec.MB-0005-2014. doi: 10.1128/microbiolspec.MB-0005-2014. Microbiol Spectr. 2015. PMID: 26185083 Free PMC article.
-
Nanoemulsion as an Effective Treatment against Human-Pathogenic Fungi.mSphere. 2019 Dec 18;4(6):e00729-19. doi: 10.1128/mSphere.00729-19. mSphere. 2019. PMID: 31852807 Free PMC article.
-
The Culture Environment Influences Both Gene Regulation and Phenotypic Heterogeneity in Escherichia coli.Front Microbiol. 2018 Aug 15;9:1739. doi: 10.3389/fmicb.2018.01739. eCollection 2018. Front Microbiol. 2018. PMID: 30158905 Free PMC article.
References
-
- Baginski, M., K. Sternal, J. Czub, and E. Borowski. 2005. Molecular modelling of membrane activity of amphotericin B, a polyene macrolide antifungal antibiotic. Acta Biochim. Pol. 52:655-658. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. - PubMed
-
- Balaban, N. Q., J. Merrin, R. Chait, L. Kowalik, and S. Leibler. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622-1625. - PubMed
-
- Basrani, B., and C. Lemonie. 2005. Chlorhexidine gluconate. Aust. Endod. J. 31:48-52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

