Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses
- PMID: 17079283
- PMCID: PMC1797448
- DOI: 10.1128/JVI.01167-06
Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses
Abstract
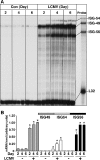
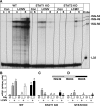
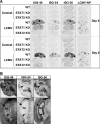
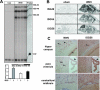
The interferon (IFN)-stimulated genes (ISGs) ISG-49, ISG-54, and ISG-56 are highly responsive to viral infection, yet the regulation and function of these genes in vivo are unknown. We examined the simultaneous regulation of these ISGs in the brains of mice during infection with either lymphocytic choriomeningitis virus (LCMV) or West Nile virus (WNV). Expression of the ISG-49 and ISG-56 genes increased significantly during LCMV infection, being widespread and localized predominantly to common as well as distinct neuronal populations. Expression of the ISG-54 gene also increased but to lower levels and with a more restricted distribution. Although expression of the ISG-49, ISG-54, and ISG-56 genes was increased in the brains of LCMV-infected STAT1 and STAT2 knockout (KO) mice, this was blunted, delayed, and restricted to the choroid plexus, meninges, and endothelium. ISG-56 protein was regulated in parallel with the corresponding RNA transcript in the brain during LCMV infection in wild-type and STAT KO mice. Similar changes in ISG-49, ISG-54, and ISG-56 RNA levels and ISG-56 protein levels were observed in the brains of wild-type mice following infection with WNV. Thus, the ISG-49, ISG-54, and ISG-56 genes are coordinately upregulated in the brain during LCMV and WNV infection; this upregulation, in the case of LCMV, was totally (neurons) or partially (non-neurons) dependent on the IFN-signaling molecules STAT1 and STAT2. These findings suggest a dominant role for the ISG-49, ISG-54, and ISG-56 genes in the host response to different viruses in the central nervous system, where, particularly in neurons, these genes may have nonredundant functions.
Figures


Similar articles
-
Differential regulation of interferon regulatory factor (IRF)-7 and IRF-9 gene expression in the central nervous system during viral infection.J Virol. 2005 Jun;79(12):7514-27. doi: 10.1128/JVI.79.12.7514-7527.2005. J Virol. 2005. PMID: 15919906 Free PMC article.
-
Mice deficient in STAT1 but not STAT2 or IRF9 develop a lethal CD4+ T-cell-mediated disease following infection with lymphocytic choriomeningitis virus.J Virol. 2012 Jun;86(12):6932-46. doi: 10.1128/JVI.07147-11. Epub 2012 Apr 11. J Virol. 2012. PMID: 22496215 Free PMC article.
-
Two Interferon-Stimulated Response Elements Cooperatively Regulate Interferon-Stimulated Gene Expression in West Nile Virus-Infected IFNAR-/- Mouse Embryo Fibroblasts.J Virol. 2021 Oct 27;95(22):e0104021. doi: 10.1128/JVI.01040-21. Epub 2021 Sep 8. J Virol. 2021. PMID: 34495694 Free PMC article.
-
LCMV and the central nervous system: uncovering basic principles of CNS physiology and virus-induced disease.Curr Top Microbiol Immunol. 2002;263:177-95. doi: 10.1007/978-3-642-56055-2_9. Curr Top Microbiol Immunol. 2002. PMID: 11987814 Review. No abstract available.
-
Inflammation on the mind: visualizing immunity in the central nervous system.Curr Top Microbiol Immunol. 2009;334:227-63. doi: 10.1007/978-3-540-93864-4_10. Curr Top Microbiol Immunol. 2009. PMID: 19521688 Free PMC article. Review.
Cited by
-
Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections.J Virol. 2010 Aug;84(16):8332-41. doi: 10.1128/JVI.02199-09. Epub 2010 Jun 9. J Virol. 2010. PMID: 20534863 Free PMC article.
-
Regulation of virus-triggered type I interferon signaling by cellular and viral proteins.Front Biol (Beijing). 2010;5(1):12-31. doi: 10.1007/s11515-010-0013-x. Epub 2010 Feb 1. Front Biol (Beijing). 2010. PMID: 32215003 Free PMC article. Review.
-
IFIT1: A dual sensor and effector molecule that detects non-2'-O methylated viral RNA and inhibits its translation.Cytokine Growth Factor Rev. 2014 Oct;25(5):543-50. doi: 10.1016/j.cytogfr.2014.05.002. Epub 2014 May 17. Cytokine Growth Factor Rev. 2014. PMID: 24909568 Free PMC article. Review.
-
Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis.PLoS Pathog. 2012;8(5):e1002712. doi: 10.1371/journal.ppat.1002712. Epub 2012 May 17. PLoS Pathog. 2012. PMID: 22615570 Free PMC article.
-
Genome-wide identification of murine interferon genes in microglial-mediated neuroinflammation in Alzheimer's disease.J Neuroimmunol. 2023 Feb 15;375:578031. doi: 10.1016/j.jneuroim.2023.578031. Epub 2023 Jan 21. J Neuroimmunol. 2023. PMID: 36708632 Free PMC article.
References
-
- Asensio, V. C., C. Kincaid, and I. L. Campbell. 1999. Chemokines and the inflammatory response to viral infection in the central nervous system with a focus on lymphocytic choriomeningitis virus. J. Neurovirol. 5:65-75. - PubMed
-
- Badley, J. E., G. A. Bishop, T. St. John, and J. A. Frelinger. 1988. A simple, rapid method for the purification of poly A+ RNA. BioTechniques 6:114-116. - PubMed
-
- Bluyssen, H. A., R. J. Vlietstra, P. W. Faber, E. M. Smit, A. Hagemeijer, and J. Trapman. 1994. Structure, chromosome localization, and regulation of expression of the interferon-regulated mouse Ifi54/Ifi56 gene family. Genomics 24:137-148. - PubMed
-
- Buchmeier, M. J., R. M. Welsh, F. J. Dutko, and M. B. Oldstone. 1980. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv. Immunol. 30:275-331. - PubMed
-
- Campbell, I. L., M. V. Hobbs, P. Kemper, and M. B. A. Oldstone. 1994. Cerebral expression of multiple cytokine genes in mice with lymphocytic choriomeningitis. J. Immunol. 152:716-723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

