Transcriptional repression of the gluconeogenic gene PEPCK by the orphan nuclear receptor SHP through inhibitory interaction with C/EBPalpha
- PMID: 17094771
- PMCID: PMC1863575
- DOI: 10.1042/BJ20061549
Transcriptional repression of the gluconeogenic gene PEPCK by the orphan nuclear receptor SHP through inhibitory interaction with C/EBPalpha
Abstract
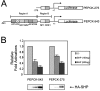
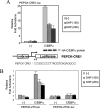
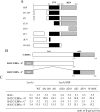
SHP (short heterodimer partner) is an orphan nuclear receptor that plays an important role in regulating glucose and lipid metabolism. A variety of transcription factors are known to regulate transcription of the PEPCK (phosphoenolpyruvate carboxykinase) gene, which encodes a rate-determining enzyme in hepatic gluconeogenesis. Previous reports identified glucocorticoid receptor and Foxo1 as novel downstream _targets regulating SHP inhibition [Borgius, Steffensen, Gustafsson and Treuter (2002) J. Biol. Chem. 277, 49761-49796; Yamagata, Daitoku, Shimamoto, Matsuzaki, Hirota, Ishida and Fukamizu (2004) J. Biol. Chem. 279, 23158-23165]. In the present paper, we show a new molecular mechanism of SHP-mediated inhibition of PEPCK transcription. We also show that the CRE1 (cAMP regulatory element 1; -99 to -76 bp relative to the transcription start site) of the PEPCK promoter is also required for the inhibitory regulation by SHP. SHP repressed C/EBPalpha (CCAAT/enhancer-binding protein alpha)-driven transcription of PEPCK through direct interaction with C/EBPalpha protein both in vitro and in vivo. The formation of an active transcriptional complex of C/EBPalpha and its binding to DNA was inhibited by SHP, resulting in the inhibition of PEPCK gene transcription. Taken together, these results suggest that SHP might regulate a level of hepatic gluconeogenesis driven by C/EBPalpha activation.
Figures
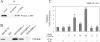
Similar articles
-
Sodium arsenite induces orphan nuclear receptor SHP gene expression via AMP-activated protein kinase to inhibit gluconeogenic enzyme gene expression.Am J Physiol Endocrinol Metab. 2008 Aug;295(2):E368-79. doi: 10.1152/ajpendo.00800.2007. Epub 2008 May 27. Am J Physiol Endocrinol Metab. 2008. PMID: 18505831
-
AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB.CRTC2 complex by orphan nuclear receptor small heterodimer partner.J Biol Chem. 2010 Oct 15;285(42):32182-91. doi: 10.1074/jbc.M110.134890. Epub 2010 Aug 5. J Biol Chem. 2010. PMID: 20688914 Free PMC article.
-
Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP.Diabetes. 2008 Feb;57(2):306-14. doi: 10.2337/db07-0381. Epub 2007 Oct 1. Diabetes. 2008. PMID: 17909097
-
Structure and function of the atypical orphan nuclear receptor small heterodimer partner.Int Rev Cytol. 2007;261:117-58. doi: 10.1016/S0074-7696(07)61003-1. Int Rev Cytol. 2007. PMID: 17560281 Review.
-
Novel concepts in insulin regulation of hepatic gluconeogenesis.Am J Physiol Endocrinol Metab. 2003 Oct;285(4):E685-92. doi: 10.1152/ajpendo.00253.2003. Am J Physiol Endocrinol Metab. 2003. PMID: 12959935 Review.
Cited by
-
Metabolic Effects of Bile Acids: Potential Role in Bariatric Surgery.Cell Mol Gastroenterol Hepatol. 2019;8(2):235-246. doi: 10.1016/j.jcmgh.2019.04.014. Epub 2019 May 7. Cell Mol Gastroenterol Hepatol. 2019. PMID: 31075353 Free PMC article. Review.
-
Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology.Nat Rev Gastroenterol Hepatol. 2022 Jul;19(7):432-450. doi: 10.1038/s41575-021-00566-7. Epub 2022 Feb 14. Nat Rev Gastroenterol Hepatol. 2022. PMID: 35165436 Review.
-
Bile acids as regulatory molecules.J Lipid Res. 2009 Aug;50(8):1509-20. doi: 10.1194/jlr.R900007-JLR200. Epub 2009 Apr 3. J Lipid Res. 2009. PMID: 19346331 Free PMC article. Review.
-
Hepatitis B virus X protein impairs hepatic insulin signaling through degradation of IRS1 and induction of SOCS3.PLoS One. 2010 Mar 23;5(3):e8649. doi: 10.1371/journal.pone.0008649. PLoS One. 2010. PMID: 20351777 Free PMC article.
-
The orphan nuclear receptor SHP inhibits apoptosis during the monocytic differentiation by inducing p21WAF1.Exp Mol Med. 2009 Jun 30;41(6):429-39. doi: 10.3858/emm.2009.41.6.048. Exp Mol Med. 2009. PMID: 19322021 Free PMC article.
References
-
- Hanson R. W., Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu. Rev. Biochem. 1997;66:581–611. - PubMed
-
- Loose D. S., Cameron D. K., Short H. P., Hanson R. W. Thyroid hormone regulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase (GTP) in rat liver. Biochemistry. 1995;24:4509–4512. - PubMed
-
- Kioussis D., Reshef L., Cohen H., Tilghman S. M., Iynedjian P. B., Ballard F. J., Hanson R. W. Alterations in translatable messenger RNA coding for phosphoenolpyruvate carboxykinase (GTP) in rat liver cytosol during deinduction. J. Biol. Chem. 1978;253:4327–4332. - PubMed
-
- Forest C. D., O'Brien R. M., Lucas P. C., Magnuson M. A., Granner D. K. Regulation of phosphoenolpyruvate carboxykinase gene expression by insulin. use of the stable transfection approach to locate an insulin responsive sequence. Mol. Endocrinol. 1990;4:1302–1310. - PubMed
-
- Scott D. K., O'Doherty R. M., Stafford J. M., Newgard C. B., Granner D. K. The repression of hormone-activated PEPCK gene expression by glucose is insulin-independent but requires glucose metabolism. J. Biol. Chem. 1998;273:24145–24151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

