Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease
- PMID: 17096854
- PMCID: PMC1657015
- DOI: 10.1186/1471-230X-6-34
Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease
Abstract
Background: Liver biopsy is considered the gold standard for assessing histologic lesions of non-alcoholic fatty liver disease (NAFLD). The aim was to develop and validate a new biomarker of non alcoholic steato hepatitis (NASH) the NashTest (NT) in patients with NAFLD.
Methods: 160 patients with NAFLD were prospectively included in a training group, 97 were included in a multicenter validation group and 383 controls. Histological diagnoses used Kleiner et al's scoring system, with 3 classes for NASH: "Not NASH", "Borderline", "NASH"). The area under the ROC curves (AUROC), sensitivity (Se), specificity (Sp), and positive and negative predictive values (PPV, NPV) were assessed.
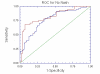
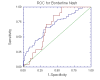
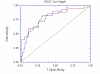
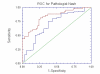
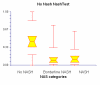
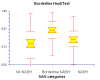
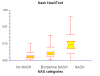
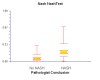
Results: NT was developed using patented algorithms combining 13 parameters: age, sex, height, weight, and serum levels of triglycerides, cholesterol, alpha2macroglobulin, apolipoprotein A1, haptoglobin, gamma-glutamyl-transpeptidase, transaminases ALT, AST, and total bilirubin. AUROCs of NT for the diagnosis of NASH in the training and validation groups were, respectively, 0.79 (95%C I 0.69-0.86) and 0.79 (95% CI 0.67-0.87; P = 0.94); for the diagnosis of borderline NASH they were: 0.69 (95% CI 0.60-0.77) and 0.69 (95% CI 0.57-0.78; P = 0.98) and for the diagnosis of no NASH, 0.77 (95% CI 0.68-0.84) and 0.83 (95% CI 0.67-0.90; P = 0.34). When the two groups were pooled together the NashTest Sp for NASH = 94% (PPV = 66%), and Se = 33% (NPV = 81%); for borderline NASH or NASH Sp = 50% (PPV = 74%) and Se = 88% (NPV = 72%).
Conclusion: In patients with non-alcoholic fatty liver disease, NashTest, a simple and non-invasive biomarker reliably predicts the presence or absence of NASH.
Figures
Similar articles
-
Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease.BMC Gastroenterol. 2006 Feb 14;6:6. doi: 10.1186/1471-230X-6-6. BMC Gastroenterol. 2006. PMID: 16503961 Free PMC article.
-
[Clinical and histological features of non-alcoholic fatty liver disease].Zhonghua Gan Zang Bing Za Zhi. 2009 Nov;17(11):812-6. Zhonghua Gan Zang Bing Za Zhi. 2009. PMID: 19958638 Chinese.
-
Usefulness of the index of NASH - ION for the diagnosis of steatohepatitis in patients with non-alcoholic fatty liver: An external validation study.Liver Int. 2018 Apr;38(4):715-723. doi: 10.1111/liv.13612. Epub 2017 Nov 3. Liver Int. 2018. PMID: 29028281
-
Characteristics and diagnosis of NAFLD/NASH.J Gastroenterol Hepatol. 2013 Dec;28 Suppl 4:64-70. doi: 10.1111/jgh.12271. J Gastroenterol Hepatol. 2013. PMID: 24251707 Review.
-
[Non-alcoholic steato-hepatitis].Praxis (Bern 1994). 2000 May 31;89(22):963-6. Praxis (Bern 1994). 2000. PMID: 10893995 Review. German.
Cited by
-
Diagnosis and evaluation of nonalcoholic fatty liver disease.Exp Diabetes Res. 2012;2012:145754. doi: 10.1155/2012/145754. Epub 2011 Oct 27. Exp Diabetes Res. 2012. PMID: 22110476 Free PMC article. Review.
-
Non-invasive methods for the diagnosis of nonalcoholic fatty liver disease.World J Hepatol. 2015 Apr 8;7(4):638-48. doi: 10.4254/wjh.v7.i4.638. World J Hepatol. 2015. PMID: 25866601 Free PMC article. Review.
-
[Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis].Internist (Berl). 2007 Feb;48(2):154-63. doi: 10.1007/s00108-006-1796-3. Internist (Berl). 2007. PMID: 17226007 Review. German.
-
Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease.Gastroenterology. 2019 Apr;156(5):1264-1281.e4. doi: 10.1053/j.gastro.2018.12.036. Epub 2019 Jan 18. Gastroenterology. 2019. PMID: 30660725 Free PMC article. Review.
-
Ultrasound elastography in patients with fatty liver disease.Radiol Bras. 2020 Jan-Feb;53(1):47-55. doi: 10.1590/0100-3984.2019.0028. Radiol Bras. 2020. PMID: 32313337 Free PMC article. Review.
References
-
- Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, Rizzetto M. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

