Vulnerability of pathogenic biofilms to Micavibrio aeruginosavorus
- PMID: 17098913
- PMCID: PMC1796979
- DOI: 10.1128/AEM.01893-06
Vulnerability of pathogenic biofilms to Micavibrio aeruginosavorus
Abstract
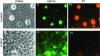
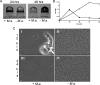
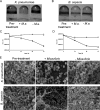
The host specificity of the gram-negative exoparasitic predatory bacterium Micavibrio aeruginosavorus was examined. M. aeruginosavorus preyed on Pseudomonas aeruginosa, as previously reported, as well as Burkholderia cepacia, Klebsiella pneumoniae, and numerous clinical isolates of these species. In a static assay, a reduction in biofilm biomass was observed as early as 3 hours after exposure to M. aeruginosavorus, and an approximately 100-fold reduction in biofilm cell viability was detected following a 24-h exposure to the predator. We observed that an initial titer of Micavibrio as low as 10 PFU/well or a time of exposure to the predator as short as 30 min was sufficient to reduce a P. aeruginosa biofilm. The ability of Micavibrio to reduce an existing biofilm was confirmed by scanning electron microscopy. In static and flow cell experiments, M. aeruginosavorus was able to modify the overall P. aeruginosa biofilm structure and markedly decreased the viability of P. aeruginosa. The altered biofilm structure was likely caused by an increase in cell-cell interactions brought about by the presence of the predator or active predation. We also conducted a screen to identify genes important for P. aeruginosa-Micavibrio interaction, but no candidates were isolated among the approximately 10,000 mutants tested.
Figures

Similar articles
-
Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus.J Appl Microbiol. 2011 Feb;110(2):431-44. doi: 10.1111/j.1365-2672.2010.04900.x. Epub 2010 Nov 29. J Appl Microbiol. 2011. PMID: 21114596
-
Susceptibility of biofilms to Bdellovibrio bacteriovorus attack.Appl Environ Microbiol. 2005 Jul;71(7):4044-51. doi: 10.1128/AEM.71.7.4044-4051.2005. Appl Environ Microbiol. 2005. PMID: 16000819 Free PMC article.
-
In Vitro Efficacy of Nonantibiotic Treatments on Biofilm Disruption of Gram-Negative Pathogens and an In Vivo Model of Infectious Endometritis Utilizing Isolates from the Equine Uterus.J Clin Microbiol. 2016 Mar;54(3):631-9. doi: 10.1128/JCM.02861-15. Epub 2015 Dec 30. J Clin Microbiol. 2016. PMID: 26719448 Free PMC article.
-
Pseudomonas aeruginosa Biofilm, a Programmed Bacterial Life for Fitness.J Microbiol Biotechnol. 2017 Jun 28;27(6):1053-1064. doi: 10.4014/jmb.1611.11056. J Microbiol Biotechnol. 2017. PMID: 28301918 Review.
-
[Interspecies interaction of bacteria and the formation of mixed (polymicrobial) biofilm].Zh Mikrobiol Epidemiol Immunobiol. 2012 Jan-Feb;(1):93-101. Zh Mikrobiol Epidemiol Immunobiol. 2012. PMID: 22442979 Review. Russian.
Cited by
-
Community and single cell analyses reveal complex predatory interactions between bacteria in high diversity systems.Nat Commun. 2021 Sep 16;12(1):5481. doi: 10.1038/s41467-021-25824-9. Nat Commun. 2021. PMID: 34531395 Free PMC article.
-
Predatory Bacteria Attenuate Klebsiella pneumoniae Burden in Rat Lungs.mBio. 2016 Nov 8;7(6):e01847-16. doi: 10.1128/mBio.01847-16. mBio. 2016. PMID: 27834203 Free PMC article.
-
Examining the efficacy of intravenous administration of predatory bacteria in rats.Sci Rep. 2017 May 12;7(1):1864. doi: 10.1038/s41598-017-02041-3. Sci Rep. 2017. PMID: 28500337 Free PMC article.
-
Anti-Proliferative and Anti-Biofilm Potentials of Bacteriocins Produced by Non-Pathogenic Enterococcus sp.Probiotics Antimicrob Proteins. 2021 Apr;13(2):571-585. doi: 10.1007/s12602-020-09711-1. Epub 2020 Oct 3. Probiotics Antimicrob Proteins. 2021. PMID: 33010007
-
Predatory bacteria as natural modulators of Vibrio parahaemolyticus and Vibrio vulnificus in seawater and oysters.Appl Environ Microbiol. 2012 Oct;78(20):7455-66. doi: 10.1128/AEM.01594-12. Epub 2012 Aug 17. Appl Environ Microbiol. 2012. PMID: 22904049 Free PMC article.
References
-
- Afinogenova, A. V., S. M. Konovalova, and V. A. Lambina. 1986. Loss of trait of species monospecificity by exoparasitic bacteria of the genus Micavibrio. Microbiology 55:377-380.
-
- Afinogenova, A. V., N. Markelova, and V. A. Lambina. 1987. Analysis of the interpopulational interactions in a 2-component bacterial system of Micavibrio admirandus-Escherichia coli. Nauchn. Dokl. Vyssh. Shk. Biol. Nauki 6:101-104. - PubMed
-
- Asaduzzaman, M., E. T. Ryan, M. John, L. Hang, A. I. Khan, A. S. Faruque, R. K. Taylor, S. B. Calderwood, and F. Qadri. 2004. The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immunoglobulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139. Infect. Immun. 72:4448-4454. - PMC - PubMed
-
- Boucher, R. C. 2004. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 23:146-158. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

