Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance
- PMID: 17126425
- PMCID: PMC2696318
- DOI: 10.1016/j.bbamcr.2006.10.001
Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance
Abstract
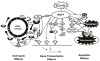
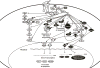
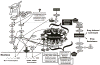
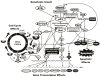
Growth factors and mitogens use the Ras/Raf/MEK/ERK signaling cascade to transmit signals from their receptors to regulate gene expression and prevent apoptosis. Some components of these pathways are mutated or aberrantly expressed in human cancer (e.g., Ras, B-Raf). Mutations also occur at genes encoding upstream receptors (e.g., EGFR and Flt-3) and chimeric chromosomal translocations (e.g., BCR-ABL) which transmit their signals through these cascades. Even in the absence of obvious genetic mutations, this pathway has been reported to be activated in over 50% of acute myelogenous leukemia and acute lymphocytic leukemia and is also frequently activated in other cancer types (e.g., breast and prostate cancers). Importantly, this increased expression is associated with a poor prognosis. The Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt pathways interact with each other to regulate growth and in some cases tumorigenesis. For example, in some cells, PTEN mutation may contribute to suppression of the Raf/MEK/ERK cascade due to the ability of activated Akt to phosphorylate and inactivate different Rafs. Although both of these pathways are commonly thought to have anti-apoptotic and drug resistance effects on cells, they display different cell lineage specific effects. For example, Raf/MEK/ERK is usually associated with proliferation and drug resistance of hematopoietic cells, while activation of the Raf/MEK/ERK cascade is suppressed in some prostate cancer cell lines which have mutations at PTEN and express high levels of activated Akt. Furthermore the Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt pathways also interact with the p53 pathway. Some of these interactions can result in controlling the activity and subcellular localization of Bim, Bak, Bax, Puma and Noxa. Raf/MEK/ERK may promote cell cycle arrest in prostate cells and this may be regulated by p53 as restoration of wild-type p53 in p53 deficient prostate cancer cells results in their enhanced sensitivity to chemotherapeutic drugs and increased expression of Raf/MEK/ERK pathway. Thus in advanced prostate cancer, it may be advantageous to induce Raf/MEK/ERK expression to promote cell cycle arrest, while in hematopoietic cancers it may be beneficial to inhibit Raf/MEK/ERK induced proliferation and drug resistance. Thus the Raf/MEK/ERK pathway has different effects on growth, prevention of apoptosis, cell cycle arrest and induction of drug resistance in cells of various lineages which may be due to the presence of functional p53 and PTEN and the expression of lineage specific factors.
Figures



Similar articles
-
Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance.Adv Enzyme Regul. 2006;46:249-79. doi: 10.1016/j.advenzreg.2006.01.004. Epub 2006 Jul 18. Adv Enzyme Regul. 2006. PMID: 16854453
-
Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy.Leukemia. 2011 Jul;25(7):1080-94. doi: 10.1038/leu.2011.66. Epub 2011 Apr 15. Leukemia. 2011. PMID: 21494257 Review.
-
Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health.Onco_target. 2011 Mar;2(3):135-64. doi: 10.18632/onco_target.240. Onco_target. 2011. PMID: 21411864 Free PMC article. Review.
-
The Raf/MEK/ERK pathway can govern drug resistance, apoptosis and sensitivity to _targeted therapy.Cell Cycle. 2010 May;9(9):1781-91. doi: 10.4161/cc.9.9.11483. Epub 2010 May 10. Cell Cycle. 2010. PMID: 20436278 Free PMC article.
-
Dominant roles of the Raf/MEK/ERK pathway in cell cycle progression, prevention of apoptosis and sensitivity to chemotherapeutic drugs.Cell Cycle. 2010 Apr 15;9(8):1629-38. doi: 10.4161/cc.9.8.11487. Epub 2010 Apr 15. Cell Cycle. 2010. PMID: 20372086 Free PMC article.
Cited by
-
CCDC134 is down-regulated in gastric cancer and its silencing promotes cell migration and invasion of GES-1 and AGS cells via the MAPK pathway.Mol Cell Biochem. 2013 Jan;372(1-2):1-8. doi: 10.1007/s11010-012-1418-4. Epub 2012 Aug 18. Mol Cell Biochem. 2013. PMID: 23070808
-
New insights into 4E-BP1-regulated translation in cancer progression and metastasis.Cancer Cell Microenviron. 2014;1(5):e331. doi: 10.14800/ccm.331. Cancer Cell Microenviron. 2014. PMID: 26005705 Free PMC article.
-
Progress in the correlation between PTPN12 gene expression and human tumors.Medicine (Baltimore). 2020 Jun 12;99(24):e20445. doi: 10.1097/MD.0000000000020445. Medicine (Baltimore). 2020. PMID: 32541467 Free PMC article.
-
Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer.Nat Rev Clin Oncol. 2016 Dec;13(12):750-765. doi: 10.1038/nrclinonc.2016.119. Epub 2016 Aug 17. Nat Rev Clin Oncol. 2016. PMID: 27531700 Review.
-
Sprouty4 negatively regulates ERK/MAPK signaling and the transition from in situ to invasive breast ductal carcinoma.PLoS One. 2021 May 28;16(5):e0252314. doi: 10.1371/journal.pone.0252314. eCollection 2021. PLoS One. 2021. PMID: 34048471 Free PMC article.
References
-
- Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004a;18:189–218. - PubMed
-
- Garnett MJ, Marais R. Guilty as charged: B-Raf is a human oncogene. Cancer Cell. 2004;6:313–319. - PubMed
-
- Yan J, Roy S, Apolloni A, Lane A, Hancock JF. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J Biol Chem. 1998;273:24052–24056. - PubMed
-
- Luo Z, Tzivion G, Belshaw PJ, Vavvas D, Marshall M, Avruch J. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature. 1996;383:181–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

