NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN
- PMID: 17218260
- PMCID: PMC1828909
- DOI: 10.1016/j.cell.2006.11.039
NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN
Abstract
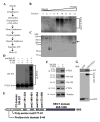
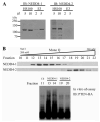
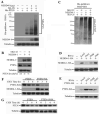
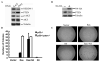
The tumor suppressor PTEN, a critical regulator for multiple cellular processes, is mutated or deleted frequently in various human cancers. Subtle reductions in PTEN expression levels have profound impacts on carcinogenesis. Here we show that PTEN level is regulated by ubiquitin-mediated proteasomal degradation, and purified its ubiquitin ligase as HECT-domain protein NEDD4-1. In cells NEDD4-1 negatively regulates PTEN stability by catalyzing PTEN polyubiquitination. Consistent with the tumor-suppressive role of PTEN, overexpression of NEDD4-1 potentiated cellular transformation. Strikingly, in a mouse cancer model and multiple human cancer samples where the genetic background of PTEN was normal but its protein levels were low, NEDD4-1 was highly expressed, suggesting that aberrant upregulation of NEDD4-1 can posttranslationally suppress PTEN in cancers. Elimination of NEDD4-1 expression inhibited xenotransplanted tumor growth in a PTEN-dependent manner. Therefore, NEDD4-1 is a potential proto-oncogene that negatively regulates PTEN via ubiquitination, a paradigm analogous to that of Mdm2 and p53.
Figures


Comment in
-
PTEN enters the nuclear age.Cell. 2007 Jan 12;128(1):25-8. doi: 10.1016/j.cell.2006.12.023. Cell. 2007. PMID: 17218252 Review.
Similar articles
-
SCF(β-TRCP)-mediated degradation of NEDD4 inhibits tumorigenesis through modulating the PTEN/Akt signaling pathway.Onco_target. 2014 Feb 28;5(4):1026-37. doi: 10.18632/onco_target.1675. Onco_target. 2014. PMID: 24657926 Free PMC article.
-
p34 is a novel regulator of the oncogenic behavior of NEDD4-1 and PTEN.Cell Death Differ. 2014 Jan;21(1):146-60. doi: 10.1038/cdd.2013.141. Epub 2013 Oct 18. Cell Death Differ. 2014. PMID: 24141722 Free PMC article.
-
Oncogenic role of the E3 ubiquitin ligase NEDD4-1, a PTEN negative regulator, in non-small-cell lung carcinomas.Am J Pathol. 2010 Nov;177(5):2622-34. doi: 10.2353/ajpath.2010.091075. Epub 2010 Oct 1. Am J Pathol. 2010. PMID: 20889565 Free PMC article.
-
NEDD4: The founding member of a family of ubiquitin-protein ligases.Gene. 2015 Feb 25;557(2):113-22. doi: 10.1016/j.gene.2014.12.020. Epub 2014 Dec 17. Gene. 2015. PMID: 25527121 Free PMC article. Review.
-
Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions.Cell Death Differ. 2010 Jan;17(1):68-77. doi: 10.1038/cdd.2009.84. Cell Death Differ. 2010. PMID: 19557014 Free PMC article. Review.
Cited by
-
Dysregulation of AKT Pathway by SMYD2-Mediated Lysine Methylation on PTEN.Neoplasia. 2015 Apr;17(4):367-73. doi: 10.1016/j.neo.2015.03.002. Neoplasia. 2015. PMID: 25925379 Free PMC article.
-
Type I γ Phosphatidylinositol Phosphate 5-Kinase i5 Controls the Ubiquitination and Degradation of the Tumor Suppressor Mitogen-inducible Gene 6.J Biol Chem. 2016 Oct 7;291(41):21461-21473. doi: 10.1074/jbc.M116.736041. Epub 2016 Aug 24. J Biol Chem. 2016. PMID: 27557663 Free PMC article.
-
Dysregulation of the Ubiquitin Proteasome System in Human Malignancies: A Window for Therapeutic Intervention.Cancers (Basel). 2021 Mar 25;13(7):1513. doi: 10.3390/cancers13071513. Cancers (Basel). 2021. PMID: 33805973 Free PMC article. Review.
-
PTEN plasticity: how the taming of a lethal gene can go too far.Trends Cell Biol. 2013 Aug;23(8):374-9. doi: 10.1016/j.tcb.2013.03.003. Epub 2013 Apr 9. Trends Cell Biol. 2013. PMID: 23578748 Free PMC article. Review.
-
Enzymatic Analysis of PTEN Ubiquitylation by WWP2 and NEDD4-1 E3 Ligases.Biochemistry. 2016 Jul 5;55(26):3658-66. doi: 10.1021/acs.biochem.6b00448. Epub 2016 Jun 22. Biochemistry. 2016. PMID: 27295432 Free PMC article.
References
-
- Adey NB, Huang L, Ormonde PA, Baumgard ML, Pero R, Byreddy DV, Tavtigian SV, Bartel PL. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 2000;60:35–37. - PubMed
-
- Bakkers J, Camacho-Carvajal M, Nowak M, Kramer C, Danger B, Hammerschmidt M. Destabilization of DeltaNp63alpha by Nedd4-mediated ubiquitination and Ubc9-mediated sumoylation, and its implications on dorsoventral patterning of the zebrafish embryo. Cell Cycle. 2005;4:790–800. - PubMed
-
- Blot V, Perugi F, Gay B, Prevost MC, Briant L, Tangy F, Abriel H, Staub O, Dokhelar MC, Pique C. Nedd4.1-mediated ubiquitination and subsequent recruitment of Tsg101 ensure HTLV-1 Gag trafficking towards the multivesicular body pathway prior to virus budding. J Cell Sci. 2004;117:2357–2367. - PubMed
-
- Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug _targets. 2005;5:3–8. - PubMed
-
- Brooks CL, Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol. 2003;15:164–171. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

