Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p
- PMID: 17339363
- PMCID: PMC1865768
- DOI: 10.1128/IAI.00054-07
Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p
Abstract
The ability of Candida albicans to invade mucosal tissues is a major virulence determinant of this organism; however, the mechanism of invasion is not understood in detail. Proteolytic breakdown of E-cadherin, the major protein in epithelial cell junctions, has been proposed as a mechanism of invasion of certain bacteria in the oral mucosa. The objectives of this study were (i) to assess whether C. albicans degrades E-cadherin expressed by oral epithelial cells in vitro; (ii) to compare the abilities of strains with different invasive potentials to degrade this protein; and (iii) to investigate fungal virulence factors responsible for E-cadherin degradation. We found that while E-cadherin gene expression was not altered, E-cadherin was proteolytically degraded during the interaction of oral epithelial cells with C. albicans. Moreover, C. albicans-mediated degradation of E-cadherin was completely inhibited in the presence of protease inhibitors. Using a three-dimensional model of the human oral mucosa, we found that E-cadherin was degraded in localized areas of tissue invasion by C. albicans. An invasion-deficient rim101-/rim101- strain was deficient in degradation of E-cadherin, and this finding suggested that proteases may depend on Rim101p for expression. Indeed, reverse transcription-PCR data indicated that expression of the SAP4, SAP5, and SAP6 genes is severely reduced in the rim101-/rim101- mutant. These SAP genes are functional Rim101p _targets, because engineered expression of SAP5 in the rim101-/rim101- strain restored E-cadherin degradation and invasion in the mucosal model. Our data support the hypothesis that there is a mechanism by which C. albicans invades mucosal tissues by promoting the proteolytic degradation of E-cadherin in epithelial adherens junctions.
Figures
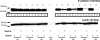

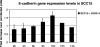



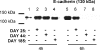

Similar articles
-
Human oral keratinocyte E-cadherin degradation by Candida albicans and Candida glabrata.J Oral Pathol Med. 2010 Mar;39(3):275-8. doi: 10.1111/j.1600-0714.2009.00866.x. J Oral Pathol Med. 2010. PMID: 20359311
-
Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs.Infect Immun. 2002 Jul;70(7):3689-700. doi: 10.1128/IAI.70.7.3689-3700.2002. Infect Immun. 2002. PMID: 12065511 Free PMC article.
-
Characterization of Candida albicans infection of an in vitro oral epithelial model using confocal laser scanning microscopy.Oral Microbiol Immunol. 2007 Jun;22(3):188-94. doi: 10.1111/j.1399-302X.2007.00344.x. Oral Microbiol Immunol. 2007. PMID: 17488445
-
Contribution of Aspartic Proteases in Candida Virulence. Protease Inhibitors against Candida Infections.Curr Protein Pept Sci. 2017;18(10):1050-1062. doi: 10.2174/1389203717666160809155749. Curr Protein Pept Sci. 2017. PMID: 27514853 Review.
-
Adaptation to environmental pH in Candida albicans and its relation to pathogenesis.Curr Genet. 2003 Oct;44(1):1-7. doi: 10.1007/s00294-003-0415-2. Epub 2003 Jun 18. Curr Genet. 2003. PMID: 12819929 Review.
Cited by
-
Systemic Infection by Non-albicans Candida Species Affects the Development of a Murine Model of Multiple Sclerosis.J Fungi (Basel). 2022 Apr 10;8(4):386. doi: 10.3390/jof8040386. J Fungi (Basel). 2022. PMID: 35448617 Free PMC article.
-
Morphogenic and genetic differences between Candida albicans strains are associated with keratomycosis virulence.Mol Vis. 2009 Jul 30;15:1476-84. Mol Vis. 2009. PMID: 19649176 Free PMC article.
-
Candida albicans ENT2 Contributes to Efficient Endocytosis, Cell Wall Integrity, Filamentation, and Virulence.mSphere. 2021 Oct 27;6(5):e0070721. doi: 10.1128/mSphere.00707-21. Epub 2021 Sep 29. mSphere. 2021. PMID: 34585966 Free PMC article.
-
Genetic variability of Candida albicans Sap8 propeptide in isolates from different types of infection.Biomed Res Int. 2015;2015:148343. doi: 10.1155/2015/148343. Epub 2015 Feb 4. Biomed Res Int. 2015. PMID: 25734055 Free PMC article.
-
In vitro evaluation of antifungal activity of monolaurin against Candida albicans biofilms.PeerJ. 2016 Jun 22;4:e2148. doi: 10.7717/peerj.2148. eCollection 2016. PeerJ. 2016. PMID: 27366648 Free PMC article.
References
-
- Bernhardt, J., K. Zimmermann, K. Schulz, M. Knoke, and H. Bernhardt. 2000. Oesophageal candidosis in intensive care patients. Mycoses 43:377-379. - PubMed
-
- Bernhardt, J., D. Herman, M. Sheridan, and R. Calderone. 2001. Adherence and invasion studies of Candida albicans strains, using in vitro models of esophageal candidiasis. J. Infect. Dis. 184:1170-1175. - PubMed
-
- Brown, A. J. P. 2001. Morphogenetic signaling pathways in Candida albicans, p. 95-106. In R. A. Calderone (ed.), Candida and candidiasis. American Society for Microbiology, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

