von Willebrand factor type D domain mutant of SVS-1/SUSD2, vWD(m), induces apoptosis in HeLa cells
- PMID: 17428257
- PMCID: PMC11159106
- DOI: 10.1111/j.1349-7006.2007.00467.x
von Willebrand factor type D domain mutant of SVS-1/SUSD2, vWD(m), induces apoptosis in HeLa cells
Abstract
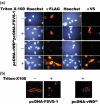
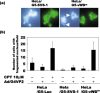
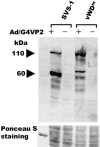
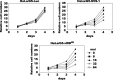
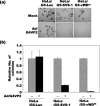
SVS-1/SUSD2 is a novel gene, which inhibits growth and reverses tumorigenic phenotypes of cancer cells in vitro. Here we report identification of a mutant of SVS-1, designated SVS-1-vWD(m), in which conserved amino acids GLLG at positions 591-594 in von Willebrand factor type D (vWD) domain are replaced by AAAA. As observed by laser confocal microscope, intracellular localization of the mutant protein has changed such that both the N-terminus and the C-terminus of SVS-1-vWD(m) were localized in the inner surface of the plasma membrane, whereas the N-terminus of SVS-1 was localized in the outer surface of the plasma membrane. Additionally, SVS-1-vWD(m) was processed much less efficiently and in a slightly different manner. In in vitro studies, adenovirus-mediated transduction of the SVS-1-vWD(m)gene induced growth suppression of HeLa cells in a dose-dependent manner, as the wild-type gene and inhibition of anchorage-independent growth. Of great interest is the finding that the mutant protein, vWD(m), but not the wild-type one induced apoptosis, as observed by nuclear as well as DNA fragmentation. Activation of caspase-3 and -9, but not caspase-8 or -12, was also demonstrated in vWD(m)-expressing cells. An inhibition of Akt phosphorylation, a major survival signaling component, also occurred in vWD(m)-expressing HeLa cells. Together these data suggest that vWD(m) induces apoptosis by inactivation of survival signaling component Akt and activation of caspase cascade (mitochondrial pathway) in HeLa cells. We propose SVS-1-vWD(m)as an alternative gene for use in developing new therapeutic strategies for the treatment of cancer.
Figures




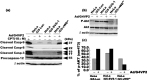
Similar articles
-
Laboratory diagnosis of von Willebrand disease type 1/2E (2A subtype IIE), type 1 Vicenza and mild type 1 caused by mutations in the D3, D4, B1-B3 and C1-C2 domains of the von Willebrand factor gene. Role of von Willebrand factor multimers and the von Willebrand factor propeptide/antigen ratio.Acta Haematol. 2009;121(2-3):128-38. doi: 10.1159/000214853. Epub 2009 Jun 8. Acta Haematol. 2009. PMID: 19506359 Review.
-
Type 2M:Milwaukee-1 von Willebrand disease: an in-frame deletion in the Cys509-Cys695 loop of the von Willebrand factor A1 domain causes deficient binding of von Willebrand factor to platelets.Blood. 1996 Oct 1;88(7):2559-68. Blood. 1996. PMID: 8839848
-
von Willebrand disease type 2A phenotypes IIC, IID and IIE: A day in the life of shear-stressed mutant von Willebrand factor.Thromb Haemost. 2014 Jul 3;112(1):96-108. doi: 10.1160/TH13-11-0902. Epub 2014 Mar 6. Thromb Haemost. 2014. PMID: 24598842
-
Evidence for the Misfolding of the A1 Domain within Multimeric von Willebrand Factor in Type 2 von Willebrand Disease.J Mol Biol. 2020 Jan 17;432(2):305-323. doi: 10.1016/j.jmb.2019.09.022. Epub 2019 Oct 17. J Mol Biol. 2020. PMID: 31628947 Free PMC article.
-
Phenotypic identification of platelet-type von Willebrand disease and its discrimination from type 2B von Willebrand disease: a question of 2B or not 2B? A story of nonidentical twins? Or two sides of a multidenominational or multifaceted primary-hemostasis coin?Semin Thromb Hemost. 2008 Feb;34(1):113-27. doi: 10.1055/s-2008-1066019. Semin Thromb Hemost. 2008. PMID: 18393148 Review.
Cited by
-
Downregulation of endometrial mesenchymal marker SUSD2 causes cell senescence and cell death in endometrial carcinoma cells.PLoS One. 2017 Aug 25;12(8):e0183681. doi: 10.1371/journal.pone.0183681. eCollection 2017. PLoS One. 2017. PMID: 28841682 Free PMC article.
-
CSBF/C10orf99, a novel potential cytokine, inhibits colon cancer cell growth through inducing G1 arrest.Sci Rep. 2014 Oct 29;4:6812. doi: 10.1038/srep06812. Sci Rep. 2014. PMID: 25351403 Free PMC article.
-
Decreased expression of Sushi Domain Containing 2 correlates to progressive features in patients with hepatocellular carcinoma.Cancer Cell Int. 2016 Feb 29;16:15. doi: 10.1186/s12935-016-0286-5. eCollection 2016. Cancer Cell Int. 2016. PMID: 26933386 Free PMC article.
-
Genome-wide association analyses in Han Chinese identify two new susceptibility loci for amyotrophic lateral sclerosis.Nat Genet. 2013 Jun;45(6):697-700. doi: 10.1038/ng.2627. Epub 2013 Apr 28. Nat Genet. 2013. PMID: 23624525
-
Loss of SUSD2 expression correlates with poor prognosis in patients with surgically resected lung adenocarcinoma.J Cancer. 2020 Jan 14;11(7):1648-1656. doi: 10.7150/jca.39319. eCollection 2020. J Cancer. 2020. PMID: 32194777 Free PMC article.
References
-
- Fang B, Roth JA. Tumor‐suppressing gene therapy. Cancer Biol Ther 2003; 2 (4 Suppl. 1): S115–21. - PubMed
-
- Hollstein M, Sidransky D, Vogelstein B et al. p53 mutations in human cancers. Science 1991; 253: 49–53. - PubMed
-
- Greenblatt MS, Bennett WP, Hollstein M et al. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 1994; 54: 4855–78. - PubMed
-
- Soussi T, Beroud C. Assessing TP53 status in human tumors to evaluate clinical outcome. Nat Rev Cancer 2001; 1: 233–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

