Interaction of a plant virus-encoded protein with the major nucleolar protein fibrillarin is required for systemic virus infection
- PMID: 17576925
- PMCID: PMC1904140
- DOI: 10.1073/pnas.0704632104
Interaction of a plant virus-encoded protein with the major nucleolar protein fibrillarin is required for systemic virus infection
Abstract
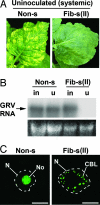
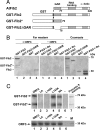
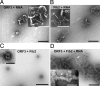
The nucleolus and specific nucleolar proteins are involved in the life cycles of some plant and animal viruses, but the functions of these proteins and of nucleolar trafficking in virus infections are largely unknown. The ORF3 protein of the plant virus, groundnut rosette virus (an umbravirus), has been shown to cycle through the nucleus, passing through Cajal bodies to the nucleolus and then exiting back into the cytoplasm. This journey is absolutely required for the formation of viral ribonucleoprotein particles (RNPs) that, themselves, are essential for the spread of the virus to noninoculated leaves of the shoot tip. Here, we show that these processes rely on the interaction of the ORF3 protein with fibrillarin, a major nucleolar protein. Silencing of the fibrillarin gene prevents long-distance movement of groundnut rosette virus but does not affect viral replication or cell-to-cell movement. Repressing fibrillarin production also localizes the ORF3 protein to multiple Cajal body-like aggregates that fail to fuse with the nucleolus. Umbraviral ORF3 protein and fibrillarin interact in vitro and, when mixed with umbravirus RNA, form an RNP complex. This complex has a filamentous structure with some regular helical features, resembling the RNP complex formed in vivo during umbravirus infection. The filaments formed in vitro are infectious when inoculated to plants, and their infectivity is resistant to RNase. These results demonstrate previously undescribed functions for fibrillarin as an essential component of translocatable viral RNPs and may have implications for other plant and animal viruses that interact with the nucleolus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A plant virus movement protein forms ringlike complexes with the major nucleolar protein, fibrillarin, in vitro.J Mol Biol. 2008 Feb 29;376(4):932-7. doi: 10.1016/j.jmb.2007.12.039. Epub 2007 Dec 28. J Mol Biol. 2008. PMID: 18199452 Free PMC article.
-
Cajal bodies and the nucleolus are required for a plant virus systemic infection.EMBO J. 2007 Apr 18;26(8):2169-79. doi: 10.1038/sj.emboj.7601674. Epub 2007 Apr 5. EMBO J. 2007. PMID: 17410203 Free PMC article.
-
Involvement of the nucleolus in plant virus systemic infection.Biochem Soc Trans. 2004 Aug;32(Pt 4):557-60. doi: 10.1042/BST0320557. Biochem Soc Trans. 2004. PMID: 15270674
-
Plant viral proteins and fibrillarin: the link to complete the infective cycle.Mol Biol Rep. 2021 May;48(5):4677-4686. doi: 10.1007/s11033-021-06401-1. Epub 2021 May 25. Mol Biol Rep. 2021. PMID: 34036480 Review.
-
Molecular biology of umbraviruses: phantom warriors.J Gen Virol. 2003 Aug;84(Pt 8):1951-1960. doi: 10.1099/vir.0.19219-0. J Gen Virol. 2003. PMID: 12867625 Review.
Cited by
-
Rice stripe virus p2 Colocalizes and Interacts with Arabidopsis Cajal Bodies and Its Domains in Plant Cells.Biomed Res Int. 2020 Jun 16;2020:5182164. doi: 10.1155/2020/5182164. eCollection 2020. Biomed Res Int. 2020. Retraction in: Biomed Res Int. 2024 Mar 20;2024:9857937. doi: 10.1155/2024/9857937 PMID: 32685498 Free PMC article. Retracted.
-
Nuclear processes associated with plant immunity and pathogen susceptibility.Brief Funct Genomics. 2015 Jul;14(4):243-52. doi: 10.1093/bfgp/elv013. Epub 2015 Apr 6. Brief Funct Genomics. 2015. PMID: 25846755 Free PMC article. Review.
-
Movement protein of hordeivirus interacts in vitro and in vivo with coilin, a major structural protein of Cajal bodies.Dokl Biochem Biophys. 2012 Jan-Feb;442:57-60. doi: 10.1134/S1607672912010164. Epub 2012 Mar 15. Dokl Biochem Biophys. 2012. PMID: 22419098 No abstract available.
-
Proteomic analysis of Sulfolobus solfataricus during Sulfolobus Turreted Icosahedral Virus infection.J Proteome Res. 2012 Feb 3;11(2):1420-32. doi: 10.1021/pr201087v. Epub 2012 Jan 24. J Proteome Res. 2012. PMID: 22217245 Free PMC article.
-
Nucleolus: the fascinating nuclear body.Histochem Cell Biol. 2008 Jan;129(1):13-31. doi: 10.1007/s00418-007-0359-6. Epub 2007 Nov 29. Histochem Cell Biol. 2008. PMID: 18046571 Free PMC article. Review.
References
-
- Olsen MOJ. In: The Nucleolus. Olsen MOJ, editor. Georgetown, TX, New York: Landes, Kluwer; 2004. pp. 329–342.
-
- Beven AF, Simpson GG, Brown JWS, Shaw PJ. J Cell Sci. 1995;108:509–518. - PubMed
-
- Cioce M, Lamond AI. Annu Rev Cell Dev Biol. 2005;21:105–131. - PubMed
-
- Venema J, Tollervey D. Annu Rev Genet. 1999;33:261–311. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

