Accelerated Abeta deposition in APPswe/PS1deltaE9 mice with hemizygous deletions of TTR (transthyretin)
- PMID: 17596449
- PMCID: PMC6672232
- DOI: 10.1523/JNEUROSCI.1919-07.2007
Accelerated Abeta deposition in APPswe/PS1deltaE9 mice with hemizygous deletions of TTR (transthyretin)
Abstract
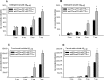
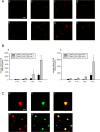
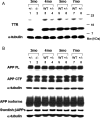
A cardinal pathological lesion of Alzheimer's disease (AD) is the deposition of amyloid beta (Abeta) in the brain. We previously reported that exposing transgenic mice harboring APPswe/PS1deltaE9 transgenes to an enriched environment resulted in reduced levels of Abeta peptides and deposition, findings that were correlated with an increase in the expression of TTR, encoding transthyretin (TTR). TTR is expressed at high levels in the choroid plexus and known to bind Abeta peptides and modulate their aggregation in vitro and in vivo. To explore the impact of TTR expression on Abeta levels and deposition in vivo, we crossed ceAPPswe/PS1deltaE9 transgenic mice to mice with genetic ablations of TTR. We now report that the levels of detergent-soluble and formic acid-soluble levels of Abeta and deposition are elevated in the brains of ceAPPswe/PS1deltaE9/TTR+/- mice compared with age-matched ceAPPswe/PS1deltaE9/TTR+/+ mice. Moreover, Abeta deposition is significantly accelerated in the hippocampus and cortex of ceAPPswe/PS1deltaE9/TTR+/- mice. Our results strongly suggest that TTR plays a critical role in modulating Abeta deposition in vivo.
Figures
Similar articles
-
Transthyretin stabilization by iododiflunisal promotes amyloid-β peptide clearance, decreases its deposition, and ameliorates cognitive deficits in an Alzheimer's disease mouse model.J Alzheimers Dis. 2014;39(2):357-70. doi: 10.3233/JAD-131355. J Alzheimers Dis. 2014. PMID: 24169237
-
Transthyretin accelerates vascular Abeta deposition in a mouse model of Alzheimer's disease.Brain Pathol. 2009 Jan;19(1):48-57. doi: 10.1111/j.1750-3639.2008.00166.x. Epub 2008 Apr 22. Brain Pathol. 2009. PMID: 18429966 Free PMC article.
-
Ube3a deficiency inhibits amyloid plaque formation in APPswe/PS1δE9 mouse model of Alzheimer's disease.Hum Mol Genet. 2017 Oct 15;26(20):4042-4054. doi: 10.1093/hmg/ddx295. Hum Mol Genet. 2017. PMID: 29016862
-
Transthyretin and the brain re-visited: is neuronal synthesis of transthyretin protective in Alzheimer's disease?Mol Neurodegener. 2011 Nov 23;6:79. doi: 10.1186/1750-1326-6-79. Mol Neurodegener. 2011. PMID: 22112803 Free PMC article. Review.
-
Transthyretin: the servant of many masters.Cell Mol Life Sci. 2009 Oct;66(19):3095-101. doi: 10.1007/s00018-009-0109-0. Epub 2009 Jul 31. Cell Mol Life Sci. 2009. PMID: 19644733 Free PMC article. Review.
Cited by
-
The choroid plexus: a missing link in our understanding of brain development and function.Physiol Rev. 2023 Jan 1;103(1):919-956. doi: 10.1152/physrev.00060.2021. Epub 2022 Sep 29. Physiol Rev. 2023. PMID: 36173801 Free PMC article. Review.
-
Cystatin C in Alzheimer's disease.Front Mol Neurosci. 2012 Jul 6;5:79. doi: 10.3389/fnmol.2012.00079. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22783166 Free PMC article.
-
Retinol-Binding Protein Interferes with Transthyretin-Mediated β-Amyloid Aggregation Inhibition.Biochemistry. 2018 Aug 21;57(33):5029-5040. doi: 10.1021/acs.biochem.8b00517. Epub 2018 Aug 1. Biochemistry. 2018. PMID: 30024734 Free PMC article.
-
Temporal gene profiling of the 5XFAD transgenic mouse model highlights the importance of microglial activation in Alzheimer's disease.Mol Neurodegener. 2014 Sep 11;9:33. doi: 10.1186/1750-1326-9-33. Mol Neurodegener. 2014. PMID: 25213090 Free PMC article.
-
Cystatin C in aging and in Alzheimer's disease.Ageing Res Rev. 2016 Dec;32:38-50. doi: 10.1016/j.arr.2016.06.003. Epub 2016 Jun 19. Ageing Res Rev. 2016. PMID: 27333827 Free PMC article. Review.
References
-
- Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988;52:487–501. - PubMed
-
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
-
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. - PubMed
-
- Chanoine JP, Alex S, Fang SL, Stone S, Leonard JL, Korhle J, Braverman LE. Role of transthyretin in the transport of thyroxine from the blood to the choroid plexus, the cerebrospinal fluid, and the brain. Endocrinology. 1992;130:933–938. - PubMed
-
- Costa DA, Cracchiolo JR, Bachstetter AD, Hughes TF, Bales KR, Paul SM, Mervis RF, Arendash GW, Potter H. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol Aging. 2006;28:831–844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
