HEF1-dependent Aurora A activation induces disassembly of the primary cilium
- PMID: 17604723
- PMCID: PMC2504417
- DOI: 10.1016/j.cell.2007.04.035
HEF1-dependent Aurora A activation induces disassembly of the primary cilium
Abstract
The mammalian cilium protrudes from the apical/lumenal surface of polarized cells and acts as a sensor of environmental cues. Numerous developmental disorders and pathological conditions have been shown to arise from defects in cilia-associated signaling proteins. Despite mounting evidence that cilia are essential sites for coordination of cell signaling, little is known about the cellular mechanisms controlling their formation and disassembly. Here, we show that interactions between the prometastatic scaffolding protein HEF1/Cas-L/NEDD9 and the oncogenic Aurora A (AurA) kinase at the basal body of cilia causes phosphorylation and activation of HDAC6, a tubulin deacetylase, promoting ciliary disassembly. We show that this pathway is both necessary and sufficient for ciliary resorption and that it constitutes an unexpected nonmitotic activity of AurA in vertebrates. Moreover, we demonstrate that small molecule inhibitors of AurA and HDAC6 selectively stabilize cilia from regulated resorption cues, suggesting a novel mode of action for these clinical agents.
Figures
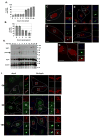
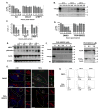
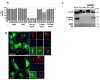
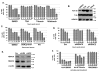

Comment in
-
The primary cilium: keeper of the key to cell division.Cell. 2007 Jun 29;129(7):1255-7. doi: 10.1016/j.cell.2007.06.018. Cell. 2007. PMID: 17604715 Review.
Similar articles
-
Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis.Mol Biol Cell. 2012 Jul;23(14):2658-70. doi: 10.1091/mbc.E11-12-1056. Epub 2012 May 23. Mol Biol Cell. 2012. PMID: 22621899 Free PMC article.
-
VHL inactivation induces HEF1 and Aurora kinase A.J Am Soc Nephrol. 2010 Dec;21(12):2041-6. doi: 10.1681/ASN.2010040345. Epub 2010 Sep 23. J Am Soc Nephrol. 2010. PMID: 20864688 Free PMC article.
-
The nephronophthisis gene product NPHP2/Inversin interacts with Aurora A and interferes with HDAC6-mediated cilia disassembly.Nephrol Dial Transplant. 2013 Nov;28(11):2744-53. doi: 10.1093/ndt/gft316. Epub 2013 Sep 11. Nephrol Dial Transplant. 2013. PMID: 24026243
-
Cell cycle progression by the repression of primary cilia formation in proliferating cells.Cell Mol Life Sci. 2013 Oct;70(20):3893-905. doi: 10.1007/s00018-013-1302-8. Epub 2013 Mar 9. Cell Mol Life Sci. 2013. PMID: 23475109 Free PMC article. Review.
-
Mechanisms for nonmitotic activation of Aurora-A at cilia.Biochem Soc Trans. 2017 Feb 8;45(1):37-49. doi: 10.1042/BST20160142. Biochem Soc Trans. 2017. PMID: 28202658 Free PMC article. Review.
Cited by
-
Phosphorylation and Ubiquitylation Regulate Protein Trafficking, Signaling, and the Biogenesis of Primary Cilia.Front Cell Dev Biol. 2021 Apr 12;9:664279. doi: 10.3389/fcell.2021.664279. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33912570 Free PMC article. Review.
-
Apical movement during interkinetic nuclear migration is a two-step process.Dev Biol. 2012 Oct 1;370(1):33-41. doi: 10.1016/j.ydbio.2012.06.031. Epub 2012 Aug 4. Dev Biol. 2012. PMID: 22884563 Free PMC article.
-
Inhibition of histone deacetylase 6 activity reduces cyst growth in polycystic kidney disease.Kidney Int. 2016 Jul;90(1):90-9. doi: 10.1016/j.kint.2016.01.026. Epub 2016 Mar 25. Kidney Int. 2016. PMID: 27165822 Free PMC article.
-
The transition zone protein Rpgrip1l regulates proteasomal activity at the primary cilium.J Cell Biol. 2015 Jul 6;210(1):115-33. doi: 10.1083/jcb.201408060. J Cell Biol. 2015. PMID: 26150391 Free PMC article.
-
Where are the limits of the centrosome?Bioarchitecture. 2016 May 3;6(3):47-52. doi: 10.1080/19490992.2016.1168957. Bioarchitecture. 2016. PMID: 27093502 Free PMC article.
References
-
- Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. - PubMed
-
- Andrews PD. Aurora kinases: shining lights on the therapeutic horizon? Oncogene. 2005;24:5005–5015. - PubMed
-
- Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr Opin Nephrol Hypertens. 2006;15:245–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

