Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells
- PMID: 17656429
- PMCID: PMC2277076
- DOI: 10.1113/jphysiol.2007.139840
Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells
Abstract
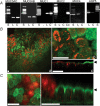
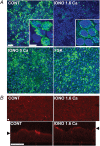
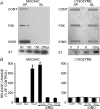
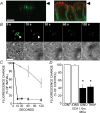
The efficiency of the mucociliary clearance (MCC) process that removes noxious materials from airway surfaces depends on the balance between mucin secretion, airway surface liquid (ASL) volume, and ciliary beating. Effective mucin dispersion into ASL requires salt and water secretion onto the mucosal surface, but how mucin secretion rate is coordinated with ion and, ultimately, water transport rates is poorly understood. Several components of MCC, including electrolyte and water transport, are regulated by nucleotides in the ASL interacting with purinergic receptors. Using polarized monolayers of airway epithelial Calu-3 cells, we investigated whether mucin secretion was accompanied by nucleotide release. Electron microscopic analyses of Calu-3 cells identified subapical granules that resembled goblet cell mucin granules. Real-time confocal microscopic analyses revealed that subapical granules, labelled with FM 1-43 or quinacrine, were competent for Ca(2+)-regulated exocytosis. Granules containing MUC5AC were apically secreted via Ca(2+)-regulated exocytosis as demonstrated by combined immunolocalization and slot blot analyses. In addition, Calu-3 cells exhibited Ca(2+)-regulated apical release of ATP and UDP-glucose, a substrate of glycosylation reactions within the secretory pathway. Neither mucin secretion nor ATP release from Calu-3 cells were affected by activation or inhibition of the cystic fibrosis transmembrane conductance regulator. In SPOC1 cells, an airway goblet cell model, purinergic P2Y(2) receptor-stimulated increase of cytosolic Ca(2+) concentration resulted in secretion of both mucins and nucleotides. Our data suggest that nucleotide release is a mechanism by which mucin-secreting goblet cells produce paracrine signals for mucin hydration within the ASL.
Figures


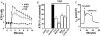
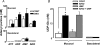
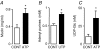
Similar articles
-
Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides.J Physiol. 2010 Jun 15;588(Pt 12):2255-67. doi: 10.1113/jphysiol.2009.186643. Epub 2010 Apr 26. J Physiol. 2010. PMID: 20421285 Free PMC article.
-
Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules.Am J Physiol Cell Physiol. 2013 May 15;304(10):C976-84. doi: 10.1152/ajpcell.00371.2012. Epub 2013 Mar 6. Am J Physiol Cell Physiol. 2013. PMID: 23467297 Free PMC article.
-
Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells.Am J Physiol. 1997 Jul;273(1 Pt 1):L201-10. doi: 10.1152/ajplung.1997.273.1.L201. Am J Physiol. 1997. PMID: 9252557
-
Airway Mucin Secretion.Ann Am Thorac Soc. 2018 Nov;15(Suppl 3):S164-S170. doi: 10.1513/AnnalsATS.201806-371AW. Ann Am Thorac Soc. 2018. PMID: 30431339 Free PMC article. Review.
-
Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling.Respir Physiol Neurobiol. 2008 Nov 30;163(1-3):208-13. doi: 10.1016/j.resp.2008.05.015. Epub 2008 May 28. Respir Physiol Neurobiol. 2008. PMID: 18635403 Free PMC article. Review.
Cited by
-
UDP-Sugars as Extracellular Signaling Molecules: Cellular and Physiologic Consequences of P2Y14 Receptor Activation.Mol Pharmacol. 2015 Jul;88(1):151-60. doi: 10.1124/mol.115.098756. Epub 2015 Mar 31. Mol Pharmacol. 2015. PMID: 25829059 Free PMC article. Review.
-
Mucus Structure, Viscoelastic Properties, and Composition in Chronic Respiratory Diseases.Int J Mol Sci. 2024 Feb 5;25(3):1933. doi: 10.3390/ijms25031933. Int J Mol Sci. 2024. PMID: 38339210 Free PMC article. Review.
-
Role of mechanical stress in regulating airway surface hydration and mucus clearance rates.Respir Physiol Neurobiol. 2008 Nov 30;163(1-3):189-201. doi: 10.1016/j.resp.2008.04.020. Epub 2008 Jun 8. Respir Physiol Neurobiol. 2008. PMID: 18585484 Free PMC article. Review.
-
Thrombin-promoted release of UDP-glucose from human astrocytoma cells.Br J Pharmacol. 2008 Apr;153(7):1528-37. doi: 10.1038/sj.bjp.0707692. Epub 2008 Jan 21. Br J Pharmacol. 2008. PMID: 18204471 Free PMC article.
-
Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways.J Biol Chem. 2009 Jul 31;284(31):20638-48. doi: 10.1074/jbc.M109.004762. Epub 2009 May 12. J Biol Chem. 2009. PMID: 19439413 Free PMC article.
References
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. - PMC - PubMed
-
- Abdullah LH, Conway JD, Cohn JA, Davis CW. Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells. Am J Physiol Lung Cell Mol Physiol. 1997;273:L201–L210. - PubMed
-
- Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts – synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271:17132–17138. - PubMed
-
- Berger JT, Voynow JA, Peters KW, Rose MC. Respiratory carcinoma cell lines. MUC genes and glycoconjugates. Am J Respir Cell Mol Biol. 1999;20:500–510. - PubMed
-
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

