Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1
- PMID: 17803352
- PMCID: PMC1961632
- DOI: 10.1371/journal.pmed.0040269
Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1
Abstract
Background: The acute respiratory distress syndrome (ARDS), a clinical complication of severe acute lung injury (ALI) in humans, is a leading cause of morbidity and mortality in critically ill patients. ALI is characterized by disruption of the lung alveolar-capillary membrane barrier and resultant pulmonary edema associated with a proteinaceous alveolar exudate. Current specific treatment strategies for ALI/ARDS are lacking. We hypothesized that mesenchymal stem cells (MSCs), with or without transfection with the vasculoprotective gene angiopoietin 1 (ANGPT1) would have beneficial effects in experimental ALI in mice.
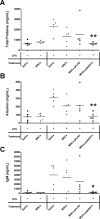
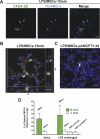
Methods and findings: Syngeneic MSCs with or without transfection with plasmid containing the human ANGPT1 gene (pANGPT1) were delivered through the right jugular vein of mice 30 min after intratracheal instillation of lipopolysaccharide (LPS) to induce lung injury. Administration of MSCs significantly reduced LPS-induced pulmonary inflammation, as reflected by reductions in total cell and neutrophil counts in bronchoalveolar lavage (BAL) fluid (53%, 95% confidence interval [CI] 7%-101%; and 60%, CI 4%-116%, respectively) as well as reducing levels of proinflammatory cytokines in both BAL fluid and lung parenchymal homogenates. Furthermore, administration of MSCs transfected with pANGPT1 resulted in nearly complete reversal of LPS-induced increases in lung permeability as assessed by reductions in IgM and albumin levels in BAL (96%, CI 6%-185%; and 74%, CI 23%-126%, respectively). Fluorescently tagged MSCs were detected in the lung tissues by confocal microscopy and flow cytometry in both naïve and LPS-injured animals up to 3 d.
Conclusions: Treatment with MSCs alone significantly reduced LPS-induced acute pulmonary inflammation in mice, while administration of pANGPT1-transfected MSCs resulted in a further improvement in both alveolar inflammation and permeability. These results suggest a potential role for cell-based ANGPT1 gene therapy to treat clinical ALI/ARDS.
Conflict of interest statement
Figures



Similar articles
-
Gene engineered mesenchymal stem cells: greater transgene expression and efficacy with minicircle vs. plasmid DNA vectors in a mouse model of acute lung injury.Stem Cell Res Ther. 2021 Mar 16;12(1):184. doi: 10.1186/s13287-021-02245-5. Stem Cell Res Ther. 2021. PMID: 33726829 Free PMC article.
-
Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice.J Pathol. 2008 Mar;214(4):472-81. doi: 10.1002/path.2302. J Pathol. 2008. PMID: 18213733
-
Angiopoietin-1 Modified Human Umbilical Cord Mesenchymal Stem Cell Therapy for Endotoxin-Induced Acute Lung Injury in Rats.Yonsei Med J. 2017 Jan;58(1):206-216. doi: 10.3349/ymj.2017.58.1.206. Yonsei Med J. 2017. PMID: 27873515 Free PMC article.
-
Genetically modified mesenchymal stem cell therapy for acute respiratory distress syndrome.Stem Cell Res Ther. 2019 Dec 16;10(1):386. doi: 10.1186/s13287-019-1518-0. Stem Cell Res Ther. 2019. PMID: 31843004 Free PMC article. Review.
-
Therapeutic implications of mesenchymal stem cells in acute lung injury/acute respiratory distress syndrome.Stem Cell Res Ther. 2013 May 2;4(3):45. doi: 10.1186/scrt193. Stem Cell Res Ther. 2013. PMID: 23673003 Free PMC article. Review.
Cited by
-
Genetically modified mesenchymal stromal cells in cancer therapy.Cytotherapy. 2016 Nov;18(11):1435-1445. doi: 10.1016/j.jcyt.2016.09.003. Cytotherapy. 2016. PMID: 27745603 Free PMC article. Review.
-
Could cancer and infection be adverse effects of mesenchymal stromal cell therapy?World J Stem Cells. 2015 Mar 26;7(2):408-17. doi: 10.4252/wjsc.v7.i2.408. World J Stem Cells. 2015. PMID: 25815124 Free PMC article. Review.
-
Regenerative medicine applications: An overview of clinical trials.Front Bioeng Biotechnol. 2022 Nov 25;10:942750. doi: 10.3389/fbioe.2022.942750. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36507264 Free PMC article. Review.
-
Acute exacerbation of idiopathic pulmonary fibrosis model by small amount of lipopolysaccharide in rats.J Clin Biochem Nutr. 2022 Mar;70(2):129-139. doi: 10.3164/jcbn.21-7. Epub 2021 Oct 1. J Clin Biochem Nutr. 2022. PMID: 35400816 Free PMC article.
-
Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats.Am J Respir Crit Care Med. 2009 Dec 1;180(11):1131-42. doi: 10.1164/rccm.200902-0179OC. Epub 2009 Aug 27. Am J Respir Crit Care Med. 2009. PMID: 19713449 Free PMC article.
References
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. - PubMed
-
- Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. - PubMed
-
- Mendez JL, Hubmayr RD. New insights into the pathology of acute respiratory failure. Curr Opin Crit Care. 2005;11:29–36. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. - PubMed
-
- Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, et al. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med. 2001;163:762–769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

