Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes
- PMID: 17938216
- PMCID: PMC2223637
- DOI: 10.1128/IAI.01039-07
Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes
Abstract
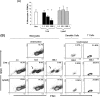
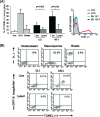
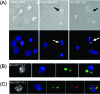
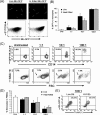
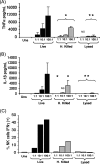
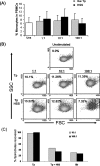
We have previously demonstrated that phagocytosed Borrelia burgdorferi induces activation programs in human peripheral blood mononuclear cells that differ qualitatively and quantitatively from those evoked by equivalent lipoprotein-rich lysates. Here we report that ingested B. burgdorferi induces significantly greater transcription of proinflammatory cytokine genes than do lysates and that live B. burgdorferi, but not B. burgdorferi lysate, is avidly internalized by monocytes, where the bacteria are completely degraded within phagolysosomes. In the course of these experiments, we discovered that live B. burgdorferi also induced a dose-dependent decrease in monocytes but not a decrease in dendritic cells or T cells and that the monocyte population displayed morphological and biochemical hallmarks of apoptosis. Particularly noteworthy was the finding that apoptotic changes occurred predominantly in monocytes that had internalized spirochetes. Abrogation of phagocytosis with cytochalasin D prevented the death response. Heat-killed B. burgdorferi, which was internalized as well as live organisms, induced a similar degree of apoptosis of monocytes but markedly less cytokine production. Surprisingly, opsonophagocytosis of Treponema pallidum did not elicit a discernible cell death response. Our combined results demonstrate that B. burgdorferi confined to phagolysosomes is a potent inducer of cytosolic signals that result in (i) production of NF-kappaB-dependent cytokines, (ii) assembly of the inflammasome and activation of caspase-1, and (iii) induction of programmed cell death. We propose that inflammation and apoptosis represent mutually reinforcing components of the immunologic arsenal that the host mobilizes to defend itself against infection with Lyme disease spirochetes.
Figures

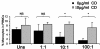
Similar articles
-
Phagocytosis of Borrelia burgdorferi and Treponema pallidum potentiates innate immune activation and induces gamma interferon production.Infect Immun. 2007 Apr;75(4):2046-62. doi: 10.1128/IAI.01666-06. Epub 2007 Jan 12. Infect Immun. 2007. PMID: 17220323 Free PMC article.
-
Human TLR8 is activated upon recognition of Borrelia burgdorferi RNA in the phagosome of human monocytes.J Leukoc Biol. 2013 Dec;94(6):1231-41. doi: 10.1189/jlb.0413206. Epub 2013 Aug 1. J Leukoc Biol. 2013. PMID: 23906644 Free PMC article. Clinical Trial.
-
Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta.PLoS Pathog. 2009 May;5(5):e1000444. doi: 10.1371/journal.ppat.1000444. Epub 2009 May 22. PLoS Pathog. 2009. PMID: 19461888 Free PMC article.
-
Mechanisms of Borrelia burgdorferi internalization and intracellular innate immune signaling.Front Cell Infect Microbiol. 2014 Dec 15;4:175. doi: 10.3389/fcimb.2014.00175. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25566512 Free PMC article. Review.
-
Actin-Dependent Regulation of Borrelia burgdorferi Phagocytosis by Macrophages.Curr Top Microbiol Immunol. 2017;399:133-154. doi: 10.1007/82_2016_26. Curr Top Microbiol Immunol. 2017. PMID: 27744511 Review.
Cited by
-
Modulation of Macrophage Redox and Apoptotic Processes to Leishmania infantum during Coinfection with the Tick-Borne Bacteria Borrelia burgdorferi.Pathogens. 2023 Sep 4;12(9):1128. doi: 10.3390/pathogens12091128. Pathogens. 2023. PMID: 37764937 Free PMC article.
-
TLR1/TLR2 heterodimers play an important role in the recognition of Borrelia spirochetes.PLoS One. 2011;6(10):e25998. doi: 10.1371/journal.pone.0025998. Epub 2011 Oct 5. PLoS One. 2011. PMID: 21998742 Free PMC article.
-
Lyme disease: the next decade.Infect Drug Resist. 2011;4:1-9. doi: 10.2147/IDR.S15653. Epub 2011 Jan 7. Infect Drug Resist. 2011. PMID: 21694904 Free PMC article.
-
Phagocytic Receptors Activate Syk and Src Signaling during Borrelia burgdorferi Phagocytosis.Infect Immun. 2017 Sep 20;85(10):e00004-17. doi: 10.1128/IAI.00004-17. Print 2017 Oct. Infect Immun. 2017. PMID: 28717031 Free PMC article.
-
The Jarisch-Herxheimer reaction associated with doxycycline in a patient with Lyme arthritis.Reumatologia. 2020;58(5):335-338. doi: 10.5114/reum.2020.99143. Epub 2020 Oct 3. Reumatologia. 2020. PMID: 33227092 Free PMC article. Review.
References
-
- Adams, J. M. 2003. Ways of dying: multiple pathways to apoptosis. Genes Dev. 172481-2495. - PubMed
-
- Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17593-623. - PubMed
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. - PubMed
-
- Albee, L., B. Shi, and H. Perlman. 2007. Aspartic protease and caspase 3/7 activation are central for macrophage apoptosis following infection with Escherichia coli. J. Leukoc. Biol. 81229-237. - PubMed
-
- Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 39286-89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 AI062439/AI/NIAID NIH HHS/United States
- AI-29735/AI/NIAID NIH HHS/United States
- AI-26756/AI/NIAID NIH HHS/United States
- AI-38894/AI/NIAID NIH HHS/United States
- M01 RR006192/RR/NCRR NIH HHS/United States
- R37 AI026756/AI/NIAID NIH HHS/United States
- R01 AI026756-20A1/AI/NIAID NIH HHS/United States
- K23 AI062439-02/AI/NIAID NIH HHS/United States
- M01RR06192/RR/NCRR NIH HHS/United States
- R01 AI026756/AI/NIAID NIH HHS/United States
- R01 AI038894/AI/NIAID NIH HHS/United States
- K23 AI-62439/AI/NIAID NIH HHS/United States
- R56 AI029735/AI/NIAID NIH HHS/United States
- R01 AI029735/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

