Infectious bronchitis viruses with a novel genomic organization
- PMID: 18045937
- PMCID: PMC2258726
- DOI: 10.1128/JVI.01694-07
Infectious bronchitis viruses with a novel genomic organization
Abstract
A number of novel infectious bronchitis viruses (IBVs) were previously identified in commercial poultry in Australia, where they caused significant economic losses. Since there has been only limited characterization of these viruses, we investigated the genomic and phenotypic differences between these novel IBVs and other, classical IBVs. The 3' 7.5 kb of the genomes of 17 Australian IBV strains were sequenced, and growth properties of 6 of the strains were compared. Comparison of sequences of the genes coding for structural and nonstructural proteins revealed the existence of two IBV genotypes: classical and novel. The genomic organization of the classical IBVs was typical of those of other group III coronaviruses: 5'-Pol-S-3a-3b-E-M-5a-5b-N-untranslated region (UTR)-3'. However, the novel IBV genotype lacked either all or most of the genes coding for nonstructural proteins at the 3' end of the genome and had a unique open reading frame, X1. The gene order was either 5'-Pol-S-X1-E-M-N-UTR-3' or 5'-Pol-S-X1-E-M-5b-N-UTR-3'. Phenotypically, novel and classical IBVs also differed; novel IBVs grew at a slower rate and reached lower titers in vitro and in vivo and were markedly less immunogenic in chicks. Although the novel IBVs induced histopathological lesions in the tracheas of infected chicks that were comparable to those induced by classical strains, they did not induce lesions in the kidneys. This study has demonstrated for the first time the existence of a naturally occurring IBV genotype devoid of some of the genes coding for nonstructural proteins and has also indicated that all of the accessory genes are dispensable for the growth of IBV and that such viruses are able to cause clinical disease and economic loss. The phylogenic differences between these novel IBVs and other avian coronaviruses suggest a reservoir host distinct from domestic poultry.
Figures
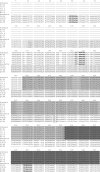
 , ORF X1;
, ORF X1;  , 3a gene;
, 3a gene;  , 3b gene;
, 3b gene;  , 3c gene.
, 3c gene.
 , ORF X1;
, ORF X1;  , 3a gene;
, 3a gene;  , 3b gene;
, 3b gene;  , 3c gene.
, 3c gene.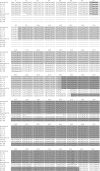
 , 5a gene;
, 5a gene;  , 5b gene.
, 5b gene.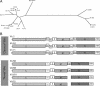
Similar articles
-
Naturally occurring recombination between distant strains of infectious bronchitis virus.Arch Virol. 2010 Oct;155(10):1581-6. doi: 10.1007/s00705-010-0731-z. Epub 2010 Jun 24. Arch Virol. 2010. PMID: 20574643 Free PMC article.
-
Origin and evolution of LX4 genotype infectious bronchitis coronavirus in China.Vet Microbiol. 2017 Jan;198:9-16. doi: 10.1016/j.vetmic.2016.11.014. Epub 2016 Nov 17. Vet Microbiol. 2017. PMID: 28062013 Free PMC article.
-
Infectious bronchitis viruses with naturally occurring genomic rearrangement and gene deletion.Arch Virol. 2011 Feb;156(2):245-52. doi: 10.1007/s00705-010-0850-6. Epub 2010 Nov 4. Arch Virol. 2011. PMID: 21049275 Free PMC article.
-
Infectious bronchitis virus in Australia: a model of coronavirus evolution - a review.Avian Pathol. 2021 Aug;50(4):295-310. doi: 10.1080/03079457.2021.1939858. Epub 2021 Jul 15. Avian Pathol. 2021. PMID: 34126817 Review.
-
Molecular evolution and emergence of avian gammacoronaviruses.Infect Genet Evol. 2012 Aug;12(6):1305-11. doi: 10.1016/j.meegid.2012.05.003. Epub 2012 May 17. Infect Genet Evol. 2012. PMID: 22609285 Free PMC article. Review.
Cited by
-
Detection of homologous recombination events in SARS-CoV-2.Biotechnol Lett. 2022 Mar;44(3):399-414. doi: 10.1007/s10529-021-03218-7. Epub 2022 Jan 17. Biotechnol Lett. 2022. PMID: 35037234 Free PMC article.
-
Delmarva (DMV/1639) Infectious Bronchitis Virus (IBV) Variants Isolated in Eastern Canada Show Evidence of Recombination.Viruses. 2019 Nov 13;11(11):1054. doi: 10.3390/v11111054. Viruses. 2019. PMID: 31766215 Free PMC article.
-
Altered pathogenicity, immunogenicity, tissue tropism and 3'-7kb region sequence of an avian infectious bronchitis coronavirus strain after serial passage in embryos.Vaccine. 2009 Jul 23;27(34):4630-40. doi: 10.1016/j.vaccine.2009.05.072. Epub 2009 Jun 11. Vaccine. 2009. PMID: 19523910 Free PMC article.
-
Recombinant infectious bronchitis virus (IBV) H120 vaccine strain expressing the hemagglutinin-neuraminidase (HN) protein of Newcastle disease virus (NDV) protects chickens against IBV and NDV challenge.Arch Virol. 2016 May;161(5):1209-16. doi: 10.1007/s00705-016-2764-4. Epub 2016 Feb 12. Arch Virol. 2016. PMID: 26873815 Free PMC article.
-
Genomic and single nucleotide polymorphism analysis of infectious bronchitis coronavirus.Infect Genet Evol. 2015 Jun;32:416-24. doi: 10.1016/j.meegid.2015.03.033. Epub 2015 Apr 3. Infect Genet Evol. 2015. PMID: 25843648 Free PMC article.
References
-
- Cavanagh, D. 2005. Coronaviridae: a review of coronaviruses and toroviruses, p. 1-54. In A. Schmidt, M. H. Wolff, and O. Weber (ed.), Coronaviruses with special emphasis on first insights concerning SARS. Birkhauser Verlag, Berlin, Germany.
-
- Cavanagh, D., P. J. Davis, J. H. Darbyshire, and R. W. Peters. 1986. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. J. Gen. Virol. 671435-1442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

