Hemopressin is an inverse agonist of CB1 cannabinoid receptors
- PMID: 18077343
- PMCID: PMC2154475
- DOI: 10.1073/pnas.0706980105
Hemopressin is an inverse agonist of CB1 cannabinoid receptors
Abstract
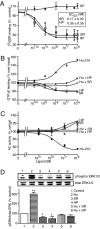
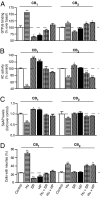
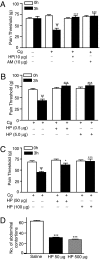
To date, the endogenous ligands described for cannabinoid receptors have been derived from membrane lipids. To identify a peptide ligand for CB(1) cannabinoid receptors, we used the recently described conformation-state sensitive antibodies and screened a panel of endogenous peptides from rodent brain or adipose tissue. This led to the identification of hemopressin (PVNFKFLSH) as a peptide ligand that selectively binds CB(1) cannabinoid receptors. We find that hemopressin is a CB(1) receptor-selective antagonist, because it is able to efficiently block signaling by CB(1) receptors but not by other members of family A G protein-coupled receptors (including the closely related CB(2) receptors). Hemopressin also behaves as an inverse agonist of CB(1) receptors, because it is able to block the constitutive activity of these receptors to the same extent as its well characterized antagonist, rimonabant. Finally, we examine the activity of hemopressin in vivo using different models of pain and find that it exhibits antinociceptive effects when administered by either intrathecal, intraplantar, or oral routes, underscoring hemopressin's therapeutic potential. These results represent a demonstration of a peptide ligand for CB(1) cannabinoid receptors that also exhibits analgesic properties. These findings are likely to have a profound impact on the development of novel therapeutics _targeting CB(1) receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
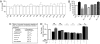
Similar articles
-
The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice.J Neurosci. 2010 May 26;30(21):7369-76. doi: 10.1523/JNEUROSCI.5455-09.2010. J Neurosci. 2010. PMID: 20505104 Free PMC article.
-
Central functional response to the novel peptide cannabinoid, hemopressin.Neuropharmacology. 2013 Aug;71:27-36. doi: 10.1016/j.neuropharm.2013.03.007. Epub 2013 Mar 28. Neuropharmacology. 2013. PMID: 23542442
-
Antinociceptive effects of central administration of the endogenous cannabinoid receptor type 1 agonist VDPVNFKLLSH-OH [(m)VD-hemopressin(α)], an N-terminally extended hemopressin peptide.J Pharmacol Exp Ther. 2014 Feb;348(2):316-23. doi: 10.1124/jpet.113.209866. Epub 2013 Dec 4. J Pharmacol Exp Ther. 2014. PMID: 24307201
-
Hemopressin as a breakthrough for the cannabinoid field.Neuropharmacology. 2021 Feb 1;183:108406. doi: 10.1016/j.neuropharm.2020.108406. Epub 2020 Nov 16. Neuropharmacology. 2021. PMID: 33212113 Free PMC article. Review.
-
Hemopressin Peptides as Modulators of the Endocannabinoid System and their Potential Applications as Therapeutic Tools.Protein Pept Lett. 2016;23(12):1045-1051. doi: 10.2174/0929866523666161007152435. Protein Pept Lett. 2016. PMID: 27748182 Review.
Cited by
-
Hemopressin and other bioactive peptides from cytosolic proteins: are these non-classical neuropeptides?AAPS J. 2010 Sep;12(3):279-89. doi: 10.1208/s12248-010-9186-0. Epub 2010 Apr 10. AAPS J. 2010. PMID: 20383670 Free PMC article. Review.
-
Intracellular peptides as natural regulators of cell signaling.J Biol Chem. 2008 Sep 5;283(36):24448-59. doi: 10.1074/jbc.M801252200. Epub 2008 Jul 10. J Biol Chem. 2008. PMID: 18617518 Free PMC article.
-
Endocannabinoid tone versus constitutive activity of cannabinoid receptors.Br J Pharmacol. 2011 Aug;163(7):1329-43. doi: 10.1111/j.1476-5381.2011.01364.x. Br J Pharmacol. 2011. PMID: 21545414 Free PMC article. Review.
-
Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity.Int J Mol Sci. 2020 Apr 7;21(7):2568. doi: 10.3390/ijms21072568. Int J Mol Sci. 2020. PMID: 32272767 Free PMC article. Review.
-
New Insights Into Peptide Cannabinoids: Structure, Biosynthesis and Signaling.Front Pharmacol. 2020 Dec 9;11:596572. doi: 10.3389/fphar.2020.596572. eCollection 2020. Front Pharmacol. 2020. PMID: 33362550 Free PMC article. Review.
References
-
- Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, Liu J, Kunos G. Pharmacol Ther. 2005;106:133–145. - PubMed
-
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Nature. 1990;346:561–564. - PubMed
-
- Munro S, Thomas KL, Abu-Shaar M. Nature. 1993;365:61–65. - PubMed
-
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, et al. Science. 2005;310:329–332. - PubMed
-
- Zhang J, Hoffert C, Va HK, Groblewski T, Ahmad S, O'Donnell D. Eur J Neurosci. 2003;17:2750–2754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

