On BC1 RNA and the fragile X mental retardation protein
- PMID: 18184799
- PMCID: PMC2206605
- DOI: 10.1073/pnas.0710991105
On BC1 RNA and the fragile X mental retardation protein
Abstract
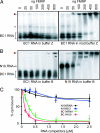
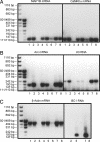
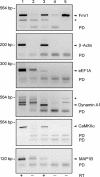
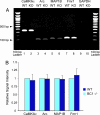
The fragile X mental retardation protein (FMRP), the functional absence of which causes fragile X syndrome, is an RNA-binding protein that has been implicated in the regulation of local protein synthesis at the synapse. The mechanism of FMRP's interaction with its _target mRNAs, however, has remained controversial. In one model, it has been proposed that BC1 RNA, a small non-protein-coding RNA that localizes to synaptodendritic domains, operates as a requisite adaptor by specifically binding to both FMRP and, via direct base-pairing, to FMRP _target mRNAs. Other models posit that FMRP interacts with its _target mRNAs directly, i.e., in a BC1-independent manner. Here five laboratories independently set out to test the BC1-FMRP model. We report that specific BC1-FMRP interactions could be documented neither in vitro nor in vivo. Interactions between BC1 RNA and FMRP _target mRNAs were determined to be of a nonspecific nature. Significantly, the association of FMRP with bona fide _target mRNAs was independent of the presence of BC1 RNA in vivo. The combined experimental evidence is discordant with a proposed scenario in which BC1 RNA acts as a bridge between FMRP and its _target mRNAs and rather supports a model in which BC1 RNA and FMRP are translational repressors that operate independently.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
On BC1 RNA and the fragile X mental retardation protein.Proc Natl Acad Sci U S A. 2008 Apr 29;105(17):E19. doi: 10.1073/pnas.0801034105. Epub 2008 Apr 15. Proc Natl Acad Sci U S A. 2008. PMID: 18417446 Free PMC article. No abstract available.
-
Reply to Bagni: On BC1 RNA and the fragile X mental retardation protein.Proc Natl Acad Sci U S A. 2008 Jun 3;105(22):E29. doi: 10.1073/pnas.0803737105. Epub 2008 May 29. Proc Natl Acad Sci U S A. 2008. PMID: 18511554 Free PMC article. No abstract available.
Similar articles
-
The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses.Cell. 2003 Feb 7;112(3):317-27. doi: 10.1016/s0092-8674(03)00079-5. Cell. 2003. PMID: 12581522
-
Regulatory BC1 RNA and the fragile X mental retardation protein: convergent functionality in brain.PLoS One. 2010 Nov 23;5(11):e15509. doi: 10.1371/journal.pone.0015509. PLoS One. 2010. PMID: 21124905 Free PMC article.
-
BC1-FMRP interaction is modulated by 2'-O-methylation: RNA-binding activity of the tudor domain and translational regulation at synapses.Nucleic Acids Res. 2012 May;40(9):4086-96. doi: 10.1093/nar/gkr1254. Epub 2012 Jan 11. Nucleic Acids Res. 2012. PMID: 22238374 Free PMC article.
-
Regulating a translational regulator: mechanisms cells use to control the activity of the fragile X mental retardation protein.Cell Mol Life Sci. 2004 Jul;61(14):1714-28. doi: 10.1007/s00018-004-4059-2. Cell Mol Life Sci. 2004. PMID: 15241549 Free PMC article. Review.
-
New insights into fragile X syndrome: from molecules to neurobehaviors.Trends Biochem Sci. 2003 Mar;28(3):152-8. doi: 10.1016/S0968-0004(03)00033-1. Trends Biochem Sci. 2003. PMID: 12633995 Review.
Cited by
-
Fragile X and APP: a Decade in Review, a Vision for the Future.Mol Neurobiol. 2019 Jun;56(6):3904-3921. doi: 10.1007/s12035-018-1344-x. Epub 2018 Sep 17. Mol Neurobiol. 2019. PMID: 30225775 Free PMC article. Review.
-
Translational control at the synapse: role of RNA regulators.Trends Biochem Sci. 2013 Jan;38(1):47-55. doi: 10.1016/j.tibs.2012.11.001. Epub 2012 Dec 4. Trends Biochem Sci. 2013. PMID: 23218750 Free PMC article. Review.
-
Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b.J Neurosci. 2010 Aug 4;30(31):10263-71. doi: 10.1523/JNEUROSCI.1125-10.2010. J Neurosci. 2010. PMID: 20685971 Free PMC article.
-
Genomic organization of eukaryotic tRNAs.BMC Genomics. 2010 Apr 28;11:270. doi: 10.1186/1471-2164-11-270. BMC Genomics. 2010. PMID: 20426822 Free PMC article.
-
Spatially restricting gene expression by local translation at synapses.Trends Neurosci. 2010 Apr;33(4):173-82. doi: 10.1016/j.tins.2010.01.005. Epub 2010 Mar 19. Trends Neurosci. 2010. PMID: 20303187 Free PMC article. Review.
References
-
- Barciszewski J, Erdmann VA, editors. Noncoding RNAs: Molecular Biology and Molecular Medicine. Georgetown, TX: Landes Bioscience; 2003.
-
- Cao X, et al. Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci. 2006;29:77–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

