Modulation of signaling pathways by RNA virus capsid proteins
- PMID: 18258415
- PMCID: PMC7127581
- DOI: 10.1016/j.cellsig.2007.12.018
Modulation of signaling pathways by RNA virus capsid proteins
Abstract
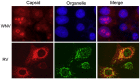
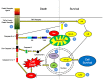
Capsid proteins are structural components of virus particles. They are nucleic acid-binding proteins whose main recognized function is to package viral genomes into protective structures called nucleocapsids. Research over the last 10 years indicates that in addition to their role as genome guardians, viral capsid proteins modulate host cell signaling networks. Disruption or alteration of intracellular signaling pathways by viral capsids may benefit replication of the virus by affecting innate immunity and in some cases, may underlie disease progression. In this review, we describe how the capsid proteins from medically relevant RNA viruses interact with host cell signaling pathways.
Figures
Similar articles
-
Non-encapsidation activities of the capsid proteins of positive-strand RNA viruses.Virology. 2013 Nov;446(1-2):123-32. doi: 10.1016/j.virol.2013.07.023. Epub 2013 Aug 27. Virology. 2013. PMID: 24074574 Free PMC article. Review.
-
Expression of flavivirus capsids enhance the cellular environment for viral replication by activating Akt-signalling pathways.Virology. 2018 Mar;516:147-157. doi: 10.1016/j.virol.2018.01.009. Epub 2018 Jan 19. Virology. 2018. PMID: 29358114
-
Acquisition of functions on the outer capsid surface during evolution of double-stranded RNA fungal viruses.PLoS Pathog. 2017 Dec 8;13(12):e1006755. doi: 10.1371/journal.ppat.1006755. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29220409 Free PMC article.
-
Structure and Noncanonical Activities of Coat Proteins of Helical Plant Viruses.Biochemistry (Mosc). 2016 Jan;81(1):1-18. doi: 10.1134/S0006297916010016. Biochemistry (Mosc). 2016. PMID: 26885578 Review.
-
Peptides as models for the structure and function of viral capsid proteins: Insights on dengue virus capsid.Biopolymers. 2013 Jul;100(4):325-36. doi: 10.1002/bip.22266. Biopolymers. 2013. PMID: 23868207
Cited by
-
Non-encapsidation activities of the capsid proteins of positive-strand RNA viruses.Virology. 2013 Nov;446(1-2):123-32. doi: 10.1016/j.virol.2013.07.023. Epub 2013 Aug 27. Virology. 2013. PMID: 24074574 Free PMC article. Review.
-
Understanding Flavivirus Capsid Protein Functions: The Tip of the Iceberg.Pathogens. 2020 Jan 5;9(1):42. doi: 10.3390/pathogens9010042. Pathogens. 2020. PMID: 31948047 Free PMC article. Review.
-
Flavivirus Infection Impairs Peroxisome Biogenesis and Early Antiviral Signaling.J Virol. 2015 Dec;89(24):12349-61. doi: 10.1128/JVI.01365-15. Epub 2015 Sep 30. J Virol. 2015. PMID: 26423946 Free PMC article.
-
Bio-Chemoinformatics-Driven Analysis of nsp7 and nsp8 Mutations and Their Effects on Viral Replication Protein Complex Stability.Curr Issues Mol Biol. 2024 Mar 18;46(3):2598-2619. doi: 10.3390/cimb46030165. Curr Issues Mol Biol. 2024. PMID: 38534781 Free PMC article.
-
A Phenome-Wide Association Study of the Effects of Fusarium graminearum Transcription Factors on Fusarium Graminearum Virus 1 Infection.Front Microbiol. 2021 Feb 11;12:622261. doi: 10.3389/fmicb.2021.622261. eCollection 2021. Front Microbiol. 2021. PMID: 33643250 Free PMC article.
References
-
- Kitler M.E., Gavinio P., Lavanchy D. Vaccine. 2002;20(Suppl 2):S5–S14. - PubMed
-
- Roulston A., Marcellus R.C., Branton P.E. Annu. Rev. Microbiol. 1999;53:577–628. - PubMed
-
- Iinuma M., Nagai Y., Maeno K., Yoshida T., Matsumoto T. J. Gen. Virol. 1971;12(3):239–247. - PubMed
-
- Kaukinen P., Vaheri A., Plyusnin A. Arch. Virol. 2005;150(9):1693–1713. - PubMed
-
- Lindenbach B.D., Thiel H.-J., Rice C.M. In: Fields Virology. Knipe D.M., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., editors. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1101–1152.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

