Enhancer of zeste homolog 2 downregulates E-cadherin by mediating histone H3 methylation in gastric cancer cells
- PMID: 18377425
- PMCID: PMC11159608
- DOI: 10.1111/j.1349-7006.2008.00743.x
Enhancer of zeste homolog 2 downregulates E-cadherin by mediating histone H3 methylation in gastric cancer cells
Abstract
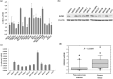
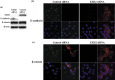
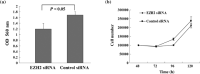
Overexpression of enhancer of zeste homolog 2 (EZH2), an epigenetic repressor, occurs in various malignancies and is associated with poor prognosis; however, the functional role of EZH2 overexpression in cancer versus non-cancerous tissue remains unclear. In this study, we found an inverse correlation between EZH2 and E-cadherin gene expression in gastric cancer cells. Knockdown of EZH2 by short interfering RNA in gastric cancer cells resulted in a restoration of the E-cadherin gene. We showed that the EZH2 complex existed with histone H3 and Lys27, which were methylated on E-cadherin promoter regions in gastric cancer cells. The restoration of E-cadherin was not involved in the change of the DNA methylation status in the E-cadherin promoter region. Immunofluorescence staining confirmed the expression of E-cadherin protein present in the cell membrane was restored after knockdown of EZH2, resulting in changing the cancer phenotype, such as its invasive capacity. In vivo, the relationship of inverse expression between EZH2 protein and E-cadherin protein was observed at the individual cellular level in gastric cancer tissue. This study provides into the mechanisms underlying the functional role of EZH2 overexpression in gastric cancer cells and a new modality of regulation of E-cadherin expression in silencing mechanisms of tumor suppressor genes. Our present study paves the way for exploring the blockade of EZH2 overexpression as a novel approach to treating cancer.
Figures


Similar articles
-
Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by increasing histone H3 methylation.J Biol Chem. 2008 Jun 20;283(25):17324-32. doi: 10.1074/jbc.M800224200. Epub 2008 Apr 22. J Biol Chem. 2008. PMID: 18430739 Free PMC article.
-
Repression of E-cadherin by the polycomb group protein EZH2 in cancer.Oncogene. 2008 Dec 11;27(58):7274-84. doi: 10.1038/onc.2008.333. Epub 2008 Sep 22. Oncogene. 2008. PMID: 18806826 Free PMC article.
-
EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin.Oncogene. 2012 Feb 2;31(5):583-94. doi: 10.1038/onc.2011.254. Epub 2011 Jun 20. Oncogene. 2012. PMID: 21685935
-
EZH2 Mediates the Regulation of S100A4 on E-cadherin Expression and the Proliferation, Migration of Gastric Cancer Cells.Hepatogastroenterology. 2015 May;62(139):737-41. Hepatogastroenterology. 2015. PMID: 26897964
-
EZH2 methyltransferase and H3K27 methylation in breast cancer.Int J Biol Sci. 2012;8(1):59-65. doi: 10.7150/ijbs.8.59. Epub 2011 Nov 18. Int J Biol Sci. 2012. PMID: 22211105 Free PMC article. Review.
Cited by
-
Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers.Nat Rev Cancer. 2010 Jul;10(7):457-69. doi: 10.1038/nrc2876. Nat Rev Cancer. 2010. PMID: 20574448 Free PMC article. Review.
-
The Attenuation of Trophoblast Invasion Caused by the Downregulation of EZH2 Is Involved in the Pathogenesis of Human Recurrent Miscarriage.Mol Ther Nucleic Acids. 2019 Mar 1;14:377-387. doi: 10.1016/j.omtn.2018.12.011. Epub 2018 Dec 22. Mol Ther Nucleic Acids. 2019. PMID: 30710891 Free PMC article.
-
Wenxia Changfu Formula () induces apoptosis of lung adenocarcinoma in a transplanted tumor model of drug-resistance nude mice.Chin J Integr Med. 2016 Oct;22(10):752-8. doi: 10.1007/s11655-015-2087-4. Epub 2015 Dec 14. Chin J Integr Med. 2016. PMID: 26666762
-
Depletion of enhancer zeste homolog 2 (EZH2) directs transcription factors associated with T cell differentiation through epigenetic regulation of Yin Yang 1(YY1) in combating non-small cell lung cancer (NSCLC).Med Oncol. 2023 May 22;40(7):185. doi: 10.1007/s12032-023-02053-2. Med Oncol. 2023. PMID: 37212947
-
The positive feedback between Snail and DAB2IP regulates EMT, invasion and metastasis in colorectal cancer.Onco_target. 2015 Sep 29;6(29):27427-39. doi: 10.18632/onco_target.4861. Onco_target. 2015. PMID: 26336990 Free PMC article.
References
-
- Takeichi M. Cadherins: a molecular family important in selective cell–cell adhesion. Annu Rev Biochem 1990; 59: 237–52. - PubMed
-
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991; 251: 1451–5. - PubMed
-
- Grunwald GB. The structural and functional analysis of cadherin calcium‐dependent cell adhesion molecules. Curr Opin Cell Biol 1993; 5: 797–805. - PubMed
-
- Pignatelli M, Vessey CJ. Adhesion molecules: novel molecular tools in tumor pathology. Hum Pathol 1994; 25: 849–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

