Vav3 oncogene activates estrogen receptor and its overexpression may be involved in human breast cancer
- PMID: 18518979
- PMCID: PMC2430719
- DOI: 10.1186/1471-2407-8-158
Vav3 oncogene activates estrogen receptor and its overexpression may be involved in human breast cancer
Abstract
Background: Our previous study revealed that Vav3 oncogene is overexpressed in human prostate cancer, activates androgen receptor, and stimulates growth in prostate cancer cells. The current study is to determine a potential role of Vav3 oncogene in human breast cancer and impact on estrogen receptor a (ERalpha)-mediated signaling axis.
Methods: Immunohistochemistry analysis was performed in 43 breast cancer specimens and western blot analysis was used for human breast cancer cell lines to determine the expression level of Vav3 protein. The impact of Vav3 on breast cancer cell growth was determined by siRNA knockdown of Vav3 expression. The role of Vav3 in ERalpha activation was examined in luciferase reporter assays. Deletion mutation analysis of Vav3 protein was performed to localize the functional domain involved in ERalpha activation. Finally, the interaction of Vav3 and ERalpha was assessed by GST pull-down analysis.
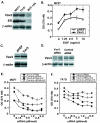
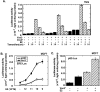
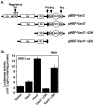
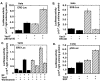
Results: We found that Vav3 was overexpressed in 81% of human breast cancer specimens, particularly in poorly differentiated lesions. Vav3 activated ERalpha partially via PI3K-Akt signaling and stimulated growth of breast cancer cells. Vav3 also potentiated EGF activity for cell growth and ERalpha activation in breast cancer cells. More interestingly, we found that Vav3 complexed with ERalpha. Consistent with its function for AR, the DH domain of Vav3 was essential for ERalpha activation.
Conclusion: Vav3 oncogene is overexpressed in human breast cancer. Vav3 complexes with ERalpha and enhances ERalpha activity. These findings suggest that Vav3 overexpression may aberrantly enhance ERalpha-mediated signaling axis and play a role in breast cancer development and/or progression.
Figures


Similar articles
-
Vav3 oncogene is overexpressed and regulates cell growth and androgen receptor activity in human prostate cancer.Mol Endocrinol. 2006 Oct;20(10):2315-25. doi: 10.1210/me.2006-0048. Epub 2006 Jun 8. Mol Endocrinol. 2006. PMID: 16762975
-
The molecular mechanism of Vav3 oncogene on upregulation of androgen receptor activity in prostate cancer cells.Int J Oncol. 2010 Mar;36(3):623-33. doi: 10.3892/ijo_00000538. Int J Oncol. 2010. PMID: 20126983
-
VAV3 mediates resistance to breast cancer endocrine therapy.Breast Cancer Res. 2014 May 28;16(3):R53. doi: 10.1186/bcr3664. Breast Cancer Res. 2014. PMID: 24886537 Free PMC article.
-
Minireview: The androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene?Mol Endocrinol. 2012 Aug;26(8):1252-67. doi: 10.1210/me.2012-1107. Epub 2012 Jun 28. Mol Endocrinol. 2012. PMID: 22745190 Free PMC article. Review.
-
VAV3 in human cancers: Mechanism and clinical implication.Pathol Res Pract. 2023 Aug;248:154681. doi: 10.1016/j.prp.2023.154681. Epub 2023 Jul 13. Pathol Res Pract. 2023. PMID: 37467637 Review.
Cited by
-
Genome-wide association study of susceptibility loci for breast cancer in Sardinian population.BMC Cancer. 2015 May 10;15:383. doi: 10.1186/s12885-015-1392-9. BMC Cancer. 2015. PMID: 25956309 Free PMC article.
-
VAV3 oncogene expression in colorectal cancer: clinical aspects and functional characterization.Sci Rep. 2015 Mar 20;5:9360. doi: 10.1038/srep09360. Sci Rep. 2015. PMID: 25791293 Free PMC article.
-
Prediction of epigenetically regulated genes in breast cancer cell lines.BMC Bioinformatics. 2010 Jun 4;11:305. doi: 10.1186/1471-2105-11-305. BMC Bioinformatics. 2010. PMID: 20525369 Free PMC article.
-
The role of Vav3 expression for inflammation and cell death during experimental myocardial infarction.Clinics (Sao Paulo). 2023 Aug 14;78:100273. doi: 10.1016/j.clinsp.2023.100273. eCollection 2023. Clinics (Sao Paulo). 2023. PMID: 37591108 Free PMC article.
-
Inhibition of gastric cancer cell growth and invasion through siRNA-mediated knockdown of guanine nucleotide exchange factor Vav3.Tumour Biol. 2014 Feb;35(2):1481-8. doi: 10.1007/s13277-013-1204-2. Epub 2013 Sep 27. Tumour Biol. 2014. PMID: 24072493
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

