YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor
- PMID: 18556781
- PMCID: PMC2438291
- DOI: 10.2353/ajpath.2008.080045
YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor
Abstract
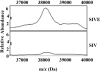
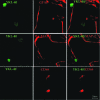
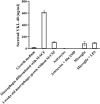
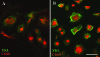
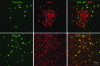
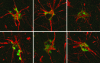
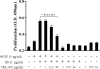
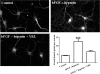
Human immunodeficiency virus encephalitis causes dementia in acquired immune deficiency syndrome patients. Using proteomic analysis of postmortem cerebrospinal fluid (CSF) and brain tissue from the simian immunodeficiency virus primate model, we demonstrate here a specific increase in YKL-40 that was tightly associated with lentiviral encephalitis. Longitudinal analysis of CSF from simian immunodeficiency virus-infected pigtailed macaques showed an increase in YKL-40 concentration 2 to 8 weeks before death from encephalitis. This increase in YKL-40 correlated with an increase in CSF viral load; it may therefore represent a biomarker for the development of encephalitis. Analysis of banked human CSF from human immunodeficiency virus-infected patients also demonstrated a correlation between YKL-40 concentration and CSF viral load. In vitro studies demonstrated increased YKL-40 expression and secretion by macrophages and microglia but not by neurons or astrocytes. We found that YKL40 displaced extracellular matrix-bound basic fibroblast growth factor (bFGF) as well as inhibited the mitogenic activity of both fibroblast growth factor receptor 1-expressing BaF3 cells and bFGF-induced axonal branching in hippocampal cultures. Taken together, these findings demonstrate that during lentiviral encephalitis, YKL-40 may interfere with the biological activity of bFGF and potentially of other heparin-binding growth factors and chemokines that can affect neuronal function or survival.
Figures




Comment in
-
YKL-40: a candidate biomarker for simian immunodeficiency virus and human immunodeficiency virus encephalitis?Am J Pathol. 2008 Jul;173(1):25-9. doi: 10.2353/ajpath.2008.080389. Am J Pathol. 2008. PMID: 18583323 Free PMC article. Review.
Similar articles
-
YKL-40: a candidate biomarker for simian immunodeficiency virus and human immunodeficiency virus encephalitis?Am J Pathol. 2008 Jul;173(1):25-9. doi: 10.2353/ajpath.2008.080389. Am J Pathol. 2008. PMID: 18583323 Free PMC article. Review.
-
Cerebrospinal Fluid Biomarkers of Simian Immunodeficiency Virus Encephalitis : CSF Biomarkers of SIV Encephalitis.J Neuroimmune Pharmacol. 2016 Jun;11(2):332-47. doi: 10.1007/s11481-016-9666-9. Epub 2016 Apr 8. J Neuroimmune Pharmacol. 2016. PMID: 27059917 Free PMC article.
-
Neuroprotective and anti-human immunodeficiency virus activity of minocycline.JAMA. 2005 Apr 27;293(16):2003-11. doi: 10.1001/jama.293.16.2003. JAMA. 2005. PMID: 15855434
-
High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis.J Virol. 1999 Dec;73(12):10480-8. doi: 10.1128/JVI.73.12.10480-10488.1999. J Virol. 1999. PMID: 10559366 Free PMC article.
-
Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies.J Neurovirol. 2008 Aug;14(4):318-26. doi: 10.1080/13550280802132857. J Neurovirol. 2008. PMID: 18780233 Free PMC article. Review.
Cited by
-
Translating the brain transcriptome in neuroAIDS: from non-human primates to humans.J Neuroimmune Pharmacol. 2012 Jun;7(2):372-9. doi: 10.1007/s11481-012-9344-5. Epub 2012 Feb 28. J Neuroimmune Pharmacol. 2012. PMID: 22367717 Free PMC article. Review.
-
YKL-40: a candidate biomarker for simian immunodeficiency virus and human immunodeficiency virus encephalitis?Am J Pathol. 2008 Jul;173(1):25-9. doi: 10.2353/ajpath.2008.080389. Am J Pathol. 2008. PMID: 18583323 Free PMC article. Review.
-
Multidimensional biomarkers for multiple system atrophy: an update and future directions.Transl Neurodegener. 2023 Jul 28;12(1):38. doi: 10.1186/s40035-023-00370-0. Transl Neurodegener. 2023. PMID: 37501056 Free PMC article. Review.
-
Cerebrospinal fluid proteomics reveals potential pathogenic changes in the brains of SIV-infected monkeys.J Proteome Res. 2009 May;8(5):2253-60. doi: 10.1021/pr800854t. J Proteome Res. 2009. PMID: 19281240 Free PMC article.
-
YKL-40 is directly produced by tumor cells and is inversely linked to EGFR in glioblastomas.Int J Clin Exp Pathol. 2010 Jan 1;3(3):226-37. Int J Clin Exp Pathol. 2010. PMID: 20224722 Free PMC article.
References
-
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. - PubMed
-
- Wiley CA, Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol. 1994;36:673–676. - PubMed
-
- Everall I, Luthert P, Lantos P. A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol. 1993;52:561–566. - PubMed
-
- Medina-Flores R, Wang G, Bissel SJ, Murphey-Corb M, Wiley CA. Destruction of extracellular matrix proteoglycans is pervasive in simian retroviral neuroinfection. Neurobiol Dis. 2004;16:604–616. - PubMed
-
- Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem. 2003;278:37753–37760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

