Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3
- PMID: 18591410
- PMCID: PMC2442632
- DOI: 10.1084/jem.20080218
Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3
Abstract
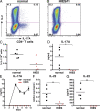
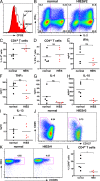
Hyper-immunoglobulin E syndrome (HIES) is a primary immune deficiency characterized by abnormal and devastating susceptibility to a narrow spectrum of infections, most commonly Staphylococcus aureus and Candida albicans. Recent investigations have identified mutations in STAT3 in the majority of HIES patients studied. Despite the identification of the genetic cause of HIES, the mechanisms underlying the pathological features of this disease remain to be elucidated. Here, we demonstrate a failure of CD4+ T cells harboring heterozygous STAT3 mutations to generate interleukin 17-secreting (i.e., T helper [Th]17) cells in vivo and in vitro due to a failure to express sufficient levels of the Th17-specific transcriptional regulator retinoid-related orphan receptor t. Because Th17 cells are enriched for cells with specificities against fungal antigens, our results may explain the pattern of infection susceptibility characteristic of patients with HIES. Furthermore, they underscore the importance of Th17 responses in normal host defense against the common pathogens S. aureus and C. albicans.
Figures

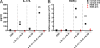
Similar articles
-
Expansion of T helper type 17 lymphocytes in patients with chronic granulomatous disease.Clin Exp Immunol. 2011 Oct;166(1):26-33. doi: 10.1111/j.1365-2249.2011.04449.x. Clin Exp Immunol. 2011. PMID: 21910722 Free PMC article.
-
Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome.J Exp Med. 2009 Jun 8;206(6):1291-301. doi: 10.1084/jem.20082767. Epub 2009 Jun 1. J Exp Med. 2009. PMID: 19487419 Free PMC article.
-
Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome.Nature. 2008 Apr 10;452(7188):773-6. doi: 10.1038/nature06764. Epub 2008 Mar 12. Nature. 2008. PMID: 18337720 Free PMC article.
-
Th17 cells, Job's syndrome and HIV: opportunities for bacterial and fungal infections.Curr Opin HIV AIDS. 2010 Mar;5(2):179-83. doi: 10.1097/COH.0b013e328335ed3e. Curr Opin HIV AIDS. 2010. PMID: 20543597 Free PMC article. Review.
-
Staphylococcus aureus and Hyper-IgE Syndrome.Int J Mol Sci. 2020 Dec 1;21(23):9152. doi: 10.3390/ijms21239152. Int J Mol Sci. 2020. PMID: 33271763 Free PMC article. Review.
Cited by
-
Identification of Novel Molecular Markers of Human Th17 Cells.Cells. 2020 Jul 3;9(7):1611. doi: 10.3390/cells9071611. Cells. 2020. PMID: 32635226 Free PMC article.
-
Dedicator of cytokinesis 8-deficient CD4+ T cells are biased to a TH2 effector fate at the expense of TH1 and TH17 cells.J Allergy Clin Immunol. 2017 Mar;139(3):933-949. doi: 10.1016/j.jaci.2016.07.016. Epub 2016 Aug 20. J Allergy Clin Immunol. 2017. PMID: 27554822 Free PMC article.
-
Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity.J Clin Invest. 2015 Jun;125(6):2211-9. doi: 10.1172/JCI78085. Epub 2015 May 11. J Clin Invest. 2015. PMID: 25961452 Free PMC article. Review.
-
Epithelial cells and innate antifungal defense.J Dent Res. 2010 Jul;89(7):666-75. doi: 10.1177/0022034510368784. Epub 2010 Apr 15. J Dent Res. 2010. PMID: 20395411 Free PMC article. Review.
-
Interleukin-17A during local and systemic Staphylococcus aureus-induced arthritis in mice.Infect Immun. 2010 Sep;78(9):3783-90. doi: 10.1128/IAI.00385-10. Epub 2010 Jun 28. Infect Immun. 2010. PMID: 20584972 Free PMC article.
References
-
- Weaver, C.T., R.D. Hatton, P.R. Mangan, and L.E. Harrington. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25:821–852. - PubMed
-
- Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. - PubMed
-
- Mangan, P.R., L.E. Harrington, D.B. O'Quinn, W.S. Helms, D.C. Bullard, C.O. Elson, R.D. Hatton, S.M. Wahl, T.R. Schoeb, and C.T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234. - PubMed
-
- Nurieva, R., X.O. Yang, G. Martinez, Y. Zhang, A.D. Panopoulos, L. Ma, K. Schluns, Q. Tian, S.S. Watowich, A.M. Jetten, and C. Dong. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 448:480–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

